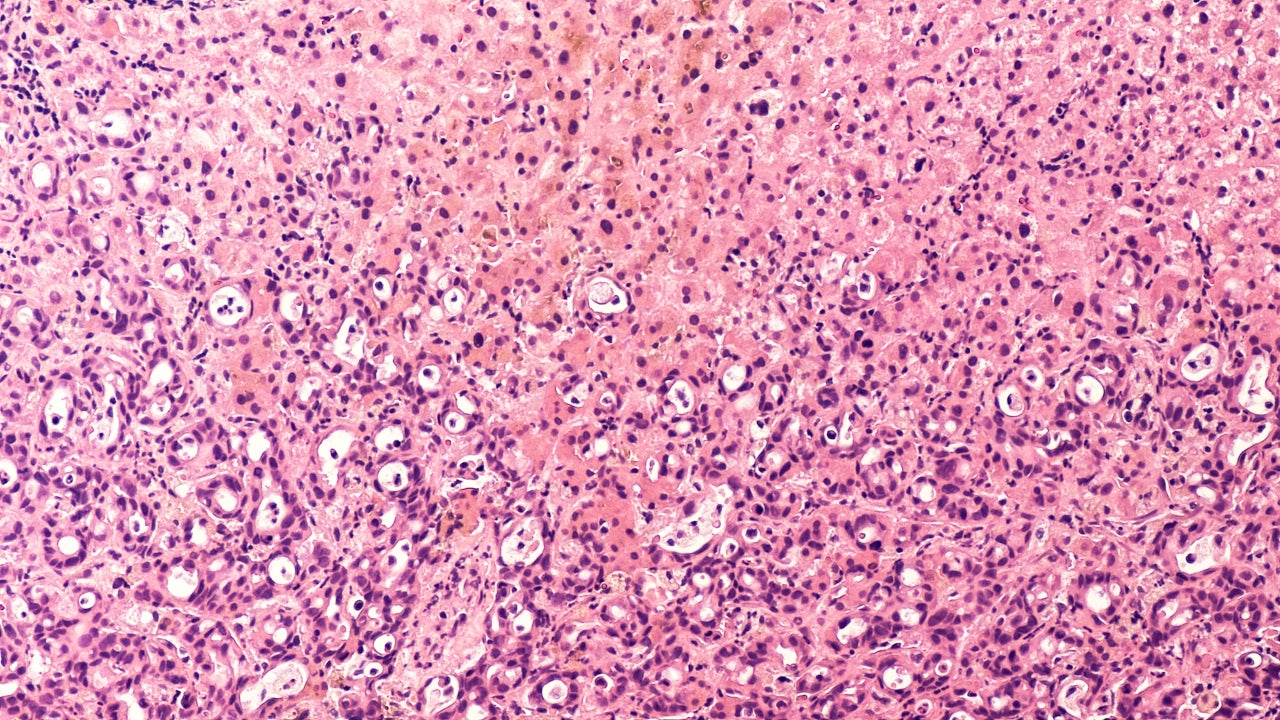
Arcus Biosciences’ Phase Ib/II adenosine receptor antagonist AB928 (etrumadenant) has a logical mechanism in first-line (1L) pancreatic ductal adenocarcinoma (PDAC), experts said. But they noted several issues that need to be addressed to maximise its efficacy potential.
The Phase Ib/II trial is studying etrumadenant in combination with Roche’s Tecentriq (atezolizumab) as well as Celgene’s Abraxane (nab-paclitaxel) plus generic gemcitabine (gem-Abraxane). But some experts suggested etrumadenant could also be combined with therapies that target upstream of the anti-inflammatory cascade. The trial should have stratified patients according to adenosine levels, they noted. While gem-Abraxane is used in 1L PDAC, immunotherapies like Tecentriq have yet to show clinical value in PDAC at all, experts added.
Phase Ib/II has coprimary endpoints investigating overall response rate (ORR) and adverse events. Experts noted that for etrumadenant to make a splash in 1L, it should show superior data over current standard of care (SOC) in the secondary endpoint of overall survival (OS). While it may be challenging to show clinical benefit in 1L, it is not impossible, owing to improved processes in finding patients to allow for earlier diagnosis, some said. Etrumadenant has a positive side-effect profile so far, though experts said the bar is low in PDAC, with the current therapies plagued by side effects.
The 290-patient Phase Ib/II Morpheus-Pancreatic Cancer trial has a primary completion date of November 2021, but the trial is investigating a variety of combinations in 1L and second-line (2L) settings for PDAC. Arcus, which has a $2.07bn market cap, did not respond to a comment request.
Etrumadenant mechanism promising, opportunities for improvement plenty
The rationale behind etrumadenant antagonising adenosine receptors is that adenosine belongs to an anti-inflammatory cascade that, if blocked, would help the immune system to potentially attack the adenocarcinoma, said adenosine receptor researcher Michail Sitkovsky, PhD, director, New England Inflammation and Tissue Protection Institute, Northeastern University, Boston, Massachusetts. When adenosine is produced and interacts with its receptors, this downregulates inflammation, which is needed to combat the adenocarcinoma, added adenosine researcher John Stagg, PhD, professor, Faculty of Pharmacy, University of Montreal, Canada. Etrumadenant targets both A2A and A2B adenosine receptors.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataWhile blocking the adenosine receptor is attractive, it is possible it could be even more attractive to block upstream of the anti-inflammatory cascade, Stagg said, adding obstructing the enzyme CD73 is another potential rate-limiting step. By blocking CD73, it would limit both adenosine production as well as its interaction with its receptor, he added. Adenosine is converted from adenosine triphosphate (ATP) to help stop inflammation, he said. This is done by CD39 depleting ATP into adenosine monophosphate (AMP), and CD73 hydrolysing AMP into adenosine, Sitkovsky explained.
On 15 January, Arcus reported preliminary data from a Phase Ib (NCT04104672) trial investigating its CD73 inhibitor AB680, also in combination with an anti-PD1 and gem-Abraxane in the same 1L PDAC setting. The Phase I portion reported of a 41% ORR in all dose-escalation cohorts, a secondary endpoint. For any early-phase trial in PDAC, any signal in the ORR endpoint is welcome for further exploration, particularly with a new mechanism; patients given systemic therapies are already very sick and have limited options, said Dr Carlos Fernández-del Castillo, director, Pancreas and Biliary Surgery Program, Massachusetts General Hospital, Boston. Safety was the primary study endpoint.
Perhaps combining etrumadenant and a CD73 inhibitor would be ideal, Stagg said. Adenosine can reach very high levels in the pancreas that could render adenosine inhibition insufficient for efficacy, he added. And so, if a CD73 inhibitor is also used, this would be a bottleneck to limit adenosine levels for AB926 to work effectively, he explained. Alternatively, patients may need to be stratified according to their adenosine levels, Sitkovsky said. Such stratification is not being performed in the Phase Ib/II.
Nevertheless, another etrumadenant efficacy obstacle is that some patients may not have enough T cells to attack the PDAC, Sitkovsky said. Even if the tumour is susceptible to attack by the etrumadenant mechanism, the relevant cells may not be there to do so, he noted. Also, there could yet be discovered gene signatures that could pinpoint which patients are more susceptible to generating high levels of adenosine, Stagg added.
Etrumadenant is being investigated with Tecentriq. Immunotherapies could upregulate A2A receptors, which argues for the combination, Stagg said. But the promise of immunotherapy in pancreatic cancer has yet to pan out because the tumour microenvironment is very immunosuppressive, noted Dr Richard Burkhardt, assistant professor of surgery, Johns Hopkins Hospital, Baltimore, Maryland. Compared with other oncology indications, PDAC does not have many tumour mutations that would make the cancer more sensitive to immunotherapy, added Dr Elisa Giovannetti, head, Molecular Mechanisms of Drug Activity, Department of Medical Oncology, VU University Medical Centre Amsterdam, the Netherlands.
Superiority needed over 1L PDAC therapies
As for 1L PDAC efficacy benchmarks for etrumadenant, it should provide superior efficacy to available therapeutic options to solidify its clinical value in this setting, experts agreed. The two therapeutic options in 1L PDAC are chemotherapy cocktail FOLFIRINOX and gem-Abraxane, said Giovannetti. FOLFIRINOX is the more efficacious option between the two as it offers a higher OS of 11 months, she added. Gem-abraxane is the third part of the etrumadenant combination; the Phase Ib/II has OS as a secondary endpoint. It is currently unclear which patients would be best suited for FOLFIRINOX or gem-Abraxane; it can only be realised once they’ve already failed the treatment, Castillo said.
Any new 1L PDAC should deliver one-year OS at least, Giovannetti added. The benchmark should be even higher at 13 or 14 months, Castillo noted, adding FOLFIRINOX was able to demonstrate a five-month superiority over its 1L SOC predecessor.
The rationale behind the Phase I/II ORR primary endpoint is that it can provide quick information if etrumadenant offers some degree of efficacy for the asset to move to a later, riskier phase of investigation, Burkhardt said. The progression-free survival (PFS) secondary endpoint is a more valuable metric for older patients, he added. PFS and quality-of-life measures help older patients have a more comfortable life, while OS is more relevant in younger people whose success benchmark is living longer, he explained. While this may be so, PFS is harder to measure in PDAC as there is the necessity of periodic CAT scans to confirm progression, Castillo said.
Still, the challenge with succeeding in PDAC overall is that most patients are nonresponsive to systemic therapeutics right from the start, as demonstrated by the slew of failed pancreatic cancer therapies, Giovannetti said. But it is not impossible to succeed, she noted, adding that there have been strides in finding patients earlier, as demonstrated by the recent five-year survival of pancreatic cancer patients increasing from 6% to 10%, according to the American Cancer Society.
Etrumadenant has data in other oncology indications such as colorectal, castrate-resistant prostate, non-small cell lung and triple-negative breast cancers. Ras protein mutation leads to increased expression of CD39 and CD73, and Ras is a shared mutation pathway between several cancer indications, Stagg said. But extrapolation of other oncology data into PDAC can be tenuous considering each indication has its own challenges, Giovannetti added. With PDAC, the tumour itself is covered with a unique stroma that guards the tumour from therapies, she explained. Even removing the stroma does not guarantee therapeutic success, she noted.
As for side effects, the Phase Ib/II coprimary endpoint, there have been no safety red flags so far with etrumadenant’s type of immunotherapeutic approach, Stagg said, pointing to side effects seen with anti-CD73 therapies. In the Phase I/Ib AB680 trial, side effects reported were as anticipated with gem-Abraxane. There was one case of Grade 2 autoimmune hepatitis but was resolved with steroid treatment. FOLFIRINOX has a poor side effect profile so 1L PDAC has a low safety bar, experts agreed.
Reynald Castaneda is Associate Editor for Clinical Trials Arena parent company GlobalData’s investigative journalism team. A version of this article originally appeared on the Insights module of GlobalData’s Pharmaceutical Intelligence Center. To access more articles like this, visit GlobalData.







Related Company Profiles
Arcus Biosciences Inc
Celgene Corp