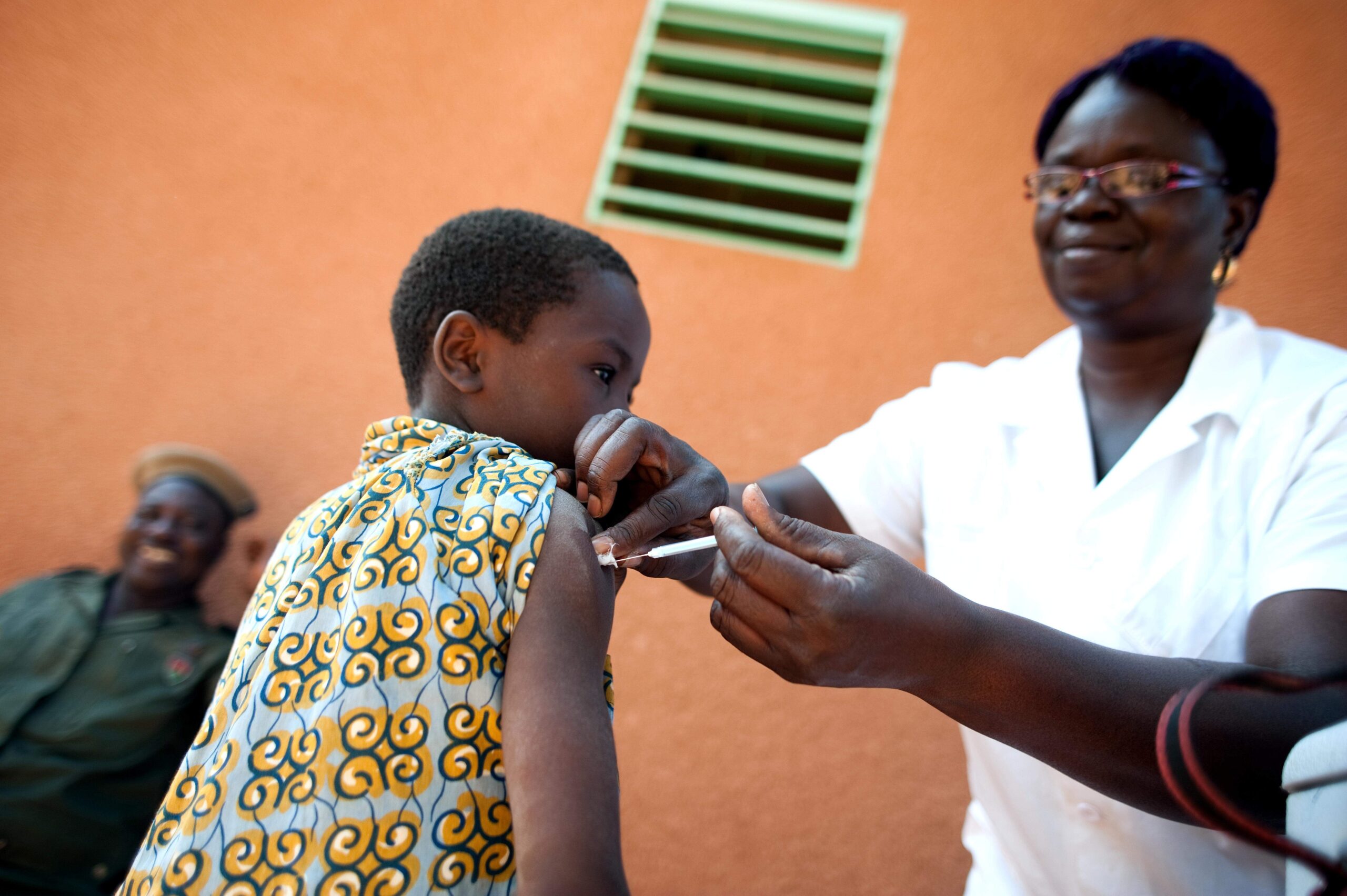
When responding to outbreaks of viral disease, public health officials are often challenged to reconcile the rapid and unpredictable nature of emerging infections with the plodding pace of vaccine development. On 22 March, researchers from the National Institute for Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) published an editorial in the Journal of the American Medical Association calling for a shift to platform vaccine manufacturing to combat emerging viral diseases. The authors believe that recent technological advances in genetic sequencing, immunogen design and synthetic vaccinology have made platform manufacturing a viable option for revolutionising the vaccine R&D process.
Historically, vaccine development is a lengthy endeavor, beginning with cultivation of an isolated virus to produce an inactivated or attenuated whole virus vaccine. Preclinical validation of the vaccine for immunogenicity and pathogenicity can take several years, followed by initial clinical testing for dosing and safety. Late-stage clinical efficacy trials pose even greater challenges, particularly for low-incidence diseases and/or those with rapidly shifting epidemiology. Overall, the time between an initial outbreak and a commercialized vaccine can span 15–20 years. Key opinion leaders (KOLs) interviewed by GlobalData stressed the need for innovative vaccine development paradigms, noting that many commonplace infections like cytomegalovirus (CMV) and respiratory syncytial virus (RSV) do not yet have vaccines, despite intense research efforts.
Platform manufacturing
Platform manufacturing applies a reproducible framework to vaccine design, allowing a fixed method for vaccine production to be applied across multiple unique pathogens. While this concept already exists in drug discovery, recent advances in genetic sequencing and epitope mapping now allow vaccine researchers to rapidly sequence a virus, synthesize DNA plasmids for preclinical testing, and manufacture genetic vectors for immunization. Drug developers establish systems of cell substrates, production protocols, and analytical assays that remain constant for any immunogen, allowing vaccine design to begin immediately once a viral sequence is obtained. When combined with streamlined animal model testing, platform technology can move a vaccine program from initial isolation of the viral pathogen to clinical trials within months, rather than years.
DNA plasmid vaccines are particularly well-suited to platform manufacturing, along with other approaches such as mRNA vaccines and virus-like particles. During the Zika virus outbreak of 2015–2016, the NIAID announced an expedited Zika vaccine development program that would leverage platform manufacturing techniques to develop a DNA vaccine. After selecting the Zika vaccine sequence, NIAID researchers successfully advanced a DNA vaccine encoding two Zika proteins to Phase I clinical trials in less than four months. The vaccine is now being evaluated in a multi-site Phase II/IIb clinical trial that is expected to conclude in 2020.
On the heels of recent epidemics such as Ebola and Zika, GlobalData believes a shift in vaccine manufacturing strategy to combat viral outbreaks has never been more necessary. The World Health Organization (WHO) recently released its second annual review of the Blueprint list of priority diseases, identifying infections most likely to cause a public health emergency due to lack of efficacious drugs or vaccines. Included among these was ‘Disease X’, an inauspicious placeholder for an unidentified future threat that nevertheless requires a prepared response. GlobalData believes that widespread adoption of platform manufacturing techniques will better position clinicians to address ‘Disease X’, as well as more established pathogens that currently lack prophylactic interventions, such as CMV or RSV.
Related Reports
GlobalData (2018). Cytomegalovirus (CMV) – Opportunity Analysis and Forecasts to 2027, to be published
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataGlobalData (2018). Expert Insight: Preparing for the Unknown: WHO Lists “Disease X” as a Priority Disease, March 2018, GDHC1665EI
GlobalData (2018). Expert Insight: With Recent Astellas Phase III Flop, a Cytomegalovirus Vaccine Continues to Elude Drug Developers, January 2018, GDHC1562EI
GlobalData (2016). Expert Insight: Zika: Short- and Long-Term Approaches to Successfully Combat the Outbreak, February 2016, GDHC1013EI




Related Company Profiles
Who LLC