AbbVie has announced its rheumatoid arthritis drug upadacitinib has achieved both primary and secondary endpoints in a Phase III study, demonstrating better results than the company’s best-selling drug Humira, a treatment for the same condition.
Upadacitinib is an orally-administered JAK1 inhibitor which is being studied as a once-daily therapy for rheumatoid arthritis, Crohn’s disease, ulcerative colitis, atopic dermatitis, psoriatic arthritis and axial spondyloarthritis (SpA). The trial examined the effect of upadacitinib in patients with an inadequate response to methotrexate, a chemotherapy agent and immune system suppressant.
The trial found that after 12 weeks of a once-daily dose of upadacitinib 15mg, 71% of patients achieved an ACR20 response, defined as an improvement in symptoms of at least 20%. This compares to 63% of patients taking 40mg Humira who achieved ACR20 response, and 36% of patients taking placebo.
Additionally, 45% of patients taking upadacitinib achieved an ACR50 (at least 50% improvement in symptoms) response, while 29% of those taking Humira and 15% of those taking placebo achieved the same. The rate of ACR70 responses was 25%, 13% and 5% respectively.
Clinical remission was achieved by 29% of patients taking upadacitinib, compared to 18% taking Humira and 6% taking placebo. The safety profile of upadacitinib was consistent with previously reported results, with no new safety signals detected. There were no cases of venous thromboembolism in any of the 1,629 participants – an effect seen in two previous trials that led to instances of death.
“These results show a significant impact on both signs and symptoms and radiographic progression compared to placebo, as well as improvements in important measures such as ACR response and low disease activity compared to adalimumab,” AbbVie vice president Dr Michael Severino said.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData“We are excited by these strong results which add to the body of evidence that support the potential of upadacitinib to be an important treatment option for patients with rheumatoid arthritis.”
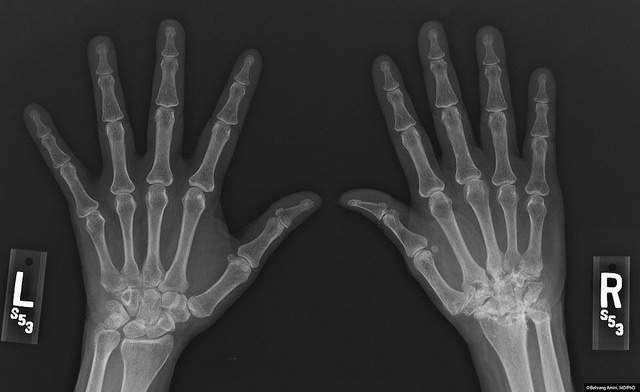
Upadacitinib was also shown to significantly inhibit radiographic progression at week 26 compared to placebo.
Rheumatoid arthritis causes pain, swelling and stiffness in joints and currently affects around 23.7 million people worldwide. Although a range of treatment options are available, many patients do not achieve clinical remission or low disease activity targets.
AbbVie is planning global regulatory submissions for upadacitinib in rheumatoid arthritis in the latter half of 2018.