Biopharmaceutical company AbbVie has announced a partnership with Voyager Therapeutics that will seek to develop a new single gene therapy for neurodegenerative conditions such as Alzheimer’s disease.
The announcement comes after a series of failed Alzheimer’s clinical trials. However, gene therapies, in which healthy genes are inserted into a patient’s cells to replace impaired or missing ones, offer a possible new route for treatment.
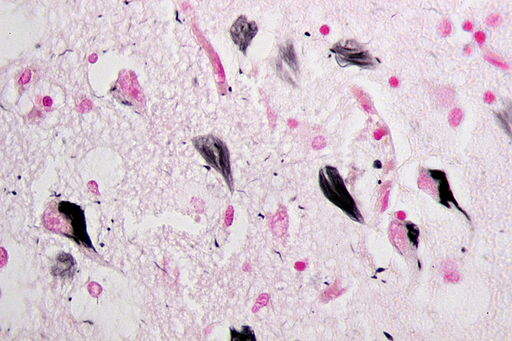
The collaboration between Voyager and AbbVie will seek to produce therapeutic antibodies that will be inserted into a patient’s cells using a benign virus. These will target clumps of the protein tau, which has been linked to Alzheimer’s disease.
In a healthy brain, tau helps create cellular stability and function, but in a diseased brain the accumulation of altered tau impairs brain function and causes neuronal cell loss. The spread of abnormal tau in the brain has been shown to correlate with progressive neurodegeneration and symptom severity.
Current weekly or biweekly biologic therapies for neurodegenerative diseases are limited by the amount of the drug that is able to make its way to the brain; however the new collaboration seeks to develop a one-time treatment to reduce tau pathology. AbbVie already has a significant body of knowledge in monoclonal antibodies, and the new partnership will allow it to also use Voyager’s gene therapy viruses platform for the creation of adeno-associated viral (AAV) vectors.
The delivery of an AAV vector antibody will alter the brain’s genetic instructions to produce anti-tau antibodies. Voyager will perform research and preclinical development of anti-tau vectorised antibodies, of which AbbVie can select one or more for Investigational New Drug-enabling studies and clinical development.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData“Voyager’s vectorised antibody platform presents an innovative approach to addressing challenges in treating neurological disorders associated with the administration of biologic therapies,” said AbbVie vice president Jim Sullivan.
“This collaboration has the potential to address the needs of patients who live with conditions such as Alzheimer’s disease, progressive supranuclear palsy and frontotemporal dementia.”
Voyager is set to receive $69 million in an upfront payment and potentially up to $155 million in preclinical and Phase I option payments, as well as up to $895 million in development and regulatory milestone payments and royalties.
The company is currently developing drugs for Huntington’s disease, amyotrophic lateral sclerosis and other brain diseases, although those treatments have yet to begin clinical studies. Its most advanced drug, a possible Parkinson’s treatment, is due to enter a Phase III trial in the second quarter of this year.







