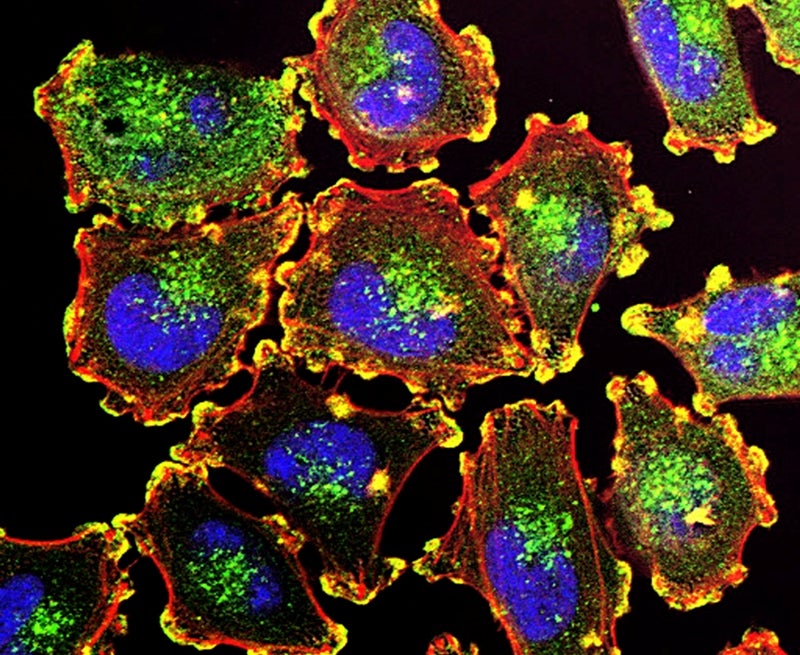
Aivita Biomedical has secured approval from the US Food and Drug Administration (FDA) for its investigational new drug (IND) application to start a Phase Ib clinical trial to assess AV-MEL-1 for the treatment of patients with metastatic melanoma.
AV-MEL-1 is developed by Aivita as part of its ‘Root of Cancer’ immunotherapy technology programme.
The company is expected to study the ‘Root of Cancer’ technology in combination with anti-PD-1 checkpoint inhibitors.
The open-label, single-arm trial aims to determine the safety of the AV-MEL-1 and anti-PD1 monoclonal antibodies combination.
It also intends to evaluate the efficacy of the treatment in around 14 to 20 patients with measurable metastatic melanoma.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataPrimary safety objective of the trial is the number of grade three to five adverse events with AV-MEL-1 plus PD-1 against PD-1 alone.
Aivita chief medical officer Dr Robert Dillman said: “As our non-responding patients all have elevated checkpoint levels, this combination therapy is a natural extension of our technology, and indeed, checkpoint inhibitors.
“Now that the limitations of anti-PD-1 therapy have been realised, many experts are touting the advantages of adding a personalised vaccine to an anti-PD-1 approach.”
Aivita previously tested its personalised patient-specific platform cancer technology in two Phase II studies that included patients with advanced melanoma.
The technology is designed to selectively target the patient’s tumour-initiating cells. It was also approved for use in a Phase III trial.
These clinical studies showed the efficacy of the technology in a randomised trial, which resulted in a 72% two-year survival rate and a 54% five-year survival rate among the patients.








