US-based Arrowhead Pharmaceuticals has completed dosing patients in a Phase I clinical trial (AROAAT1001) of drug candidate ARO-AAT for the treatment of a liver disease caused due to of alpha-1 antitrypsin deficiency (AATD).
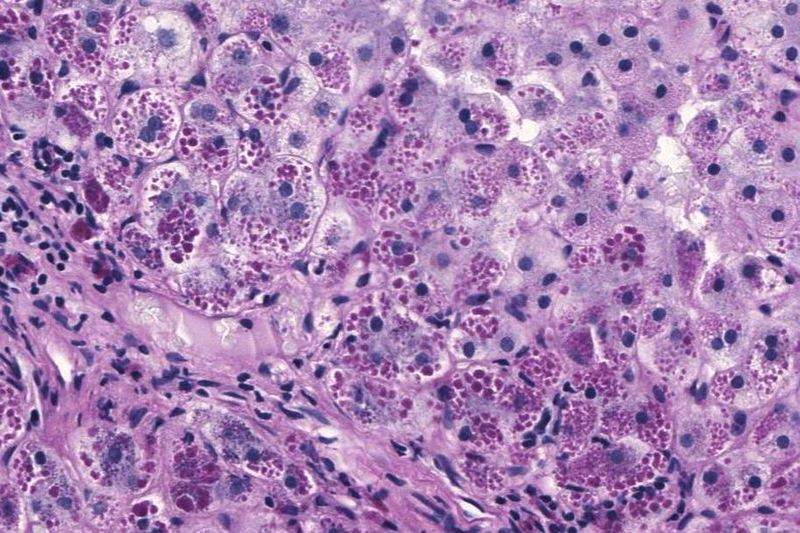
AATD is known to be a rare genetic condition that results in severe damage to the liver and lungs.
Arrowhead is developing ARO-AAT as a second-generation, subcutaneous RNA interference (RNAi) therapeutic.
The drug candidate is designed to reduce hepatic production of the mutant alpha-1 antitrypsin (Z-AAT) protein. This decrease in the production is expected to stop the liver disease progression and potentially facilitate its regeneration and repair.
AROAAT1001 is a single and multiple-ascending dose Phase I trial being conducted to investigate the safety and tolerability of ARO-AAT in healthy adult volunteers.
In addition, the trial will monitor pharmacokinetics and impact of the therapeutic on serum alpha-1 antitrypsin levels.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataThe trial comprises seven cohorts. Patients are administered with a single dose of ARO-AAT, placebo, or three monthly ARO-AAT doses of 35mg (single dose only), 100mg, 200mg, or 300mg.
While the company planned for additional cohorts at a 400mg dose, they were considered unnecessary after observing the candidate’s activity at lower doses.
The primary outcome measure of the study is proportion of subjects who experienced treatment-related adverse events (AEs).
Furthermore, the trial will evaluate secondary outcome measures such as percentage change in serum alpha-1 antitrypsin (AAT) levels, pharmacokinetics, time to maximum plasma concentration and duration of response of serum AAT levels.
Arrowhead closed enrolment for the AROAAT1001 trial in June this year. The study is expected to be completed by February next year.






