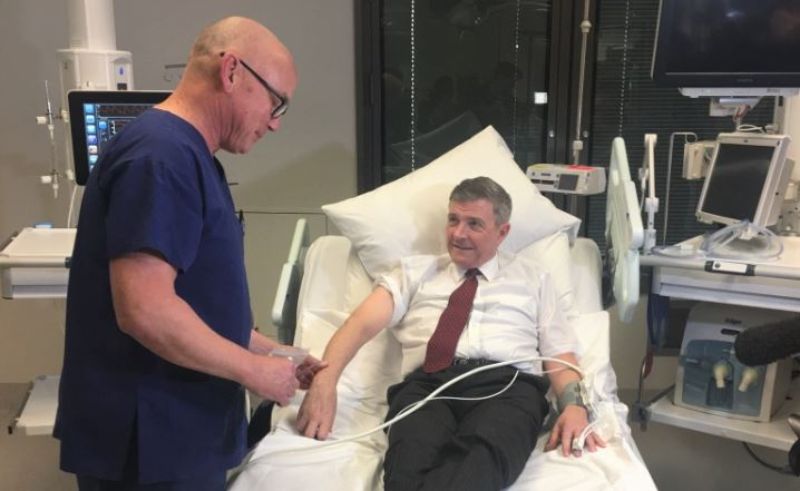
The first clinical trials of a Covid-19 vaccine developed in Australia have commenced at Royal Adelaide Hospital’s PARC Clinical Research facility.
Named COVAX-19, the targeted vaccine has been developed by company Vaxine and researchers at Flinders University.
During the first part of the trial, 40 pre-screened candidates aged between 18 and 65 will be administered with two doses of the vaccine or a placebo three weeks apart.
The aim of the trial is to assess the Covid-19 vaccine’s initial safety and immune response.
The production of antibodies and other immune responses will be analysed two weeks following the second injection, with the data expected to be made available in approximately two months.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataBlood tests will be performed to determine protective antibody and T-cell responses.
Trial principal investigator professor David Gordon said: “This Covid-19 vaccine has already shown promising results in animal models, so it is exciting to take this national breakthrough to human trials in Adelaide.”
The vaccine model was created using artificial intelligence (AI) technology with received support from the US National Institutes of Health’s National Institute of Allergy and Infectious Diseases (NIAID).
Royal Adelaide Hospital clinical immunologist Dr Pravin Hissaria said: “Now, members of the public have stood up to be among the first people in the world to receive a trial vaccine. Their actions could help us find a permanent solution to this devastating global pandemic.”
Following health checks on the first volunteer, the remaining participants will receive the vaccine at the PARC Clinical Research facility. After the evaluations, Phase II trials will be planned for additional testing.
In May this year, US-based biotechnology firm Novavax started enrolling participants in a Phase I/II clinical trial of its Covid-19 vaccine candidate, NVX‑CoV2373, in Australia.







