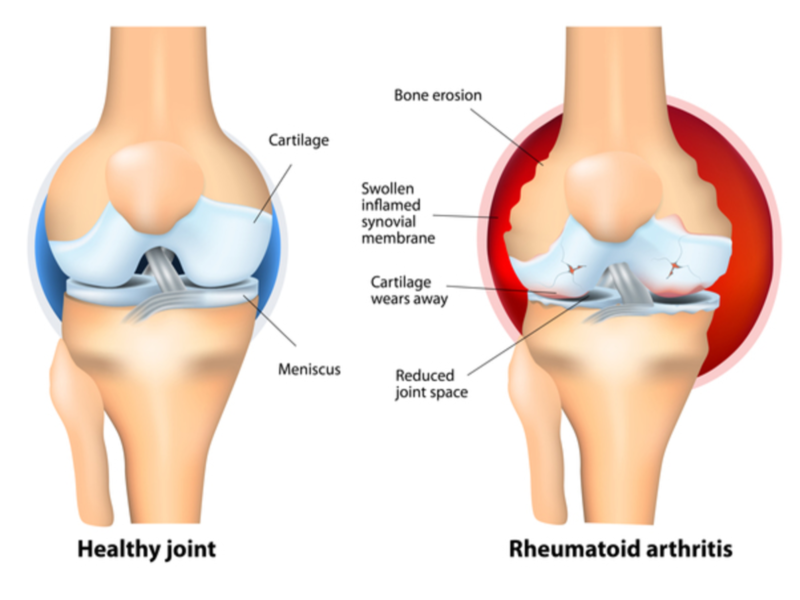
Israeli biotechnology firm Can-Fite BioPharma has started patient enrolment and dosing in a Phase III clinical trial (ACRobat) of its drug candidate Piclidenoson (CF101) for the treatment of rheumatoid arthritis.
Piclidenoson is an oral, small molecule agonist of A3 adenosine receptor
(A3AR) being developed to treat autoimmune inflammatory diseases.
In the Phase III trial, the drug candidate will be assessed as a first line treatment for the replacement of existing standard of care drug Methotrexate (MTX) for rheumatoid arthritis.
Reportedly, 50% of patients discontinue treatment with MTX due to drug intolerance, minor and major side-effects and lack of efficacy.
In previous Phase III trials with subjects who did not undergo prior systemic therapy, Piclidenoson was reported to have demonstrated favourable efficacy comparable to MTX.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataCan-Fite CEO Dr Pnina Fishman said: “We believe Piclidenoson is a potentially superior option to the standard of care, MTX, and we believe that rheumatologists are looking for a safer and more effective alternative.”
The randomised, double-blind, active and placebo-controlled ACRobat trial will investigate the long-term safety and efficacy of 1mg and 2mg twice-a-day dose of Piclidenoson in 500 participants for a period of 24 weeks at centres across Europe, Israel and Canada.
ACRobat’s primary endpoint of low disease activity following 12 weeks of treatment with Piclidenoson will be compared to once weekly dose of MTX.
Next year, the firm plans to further study Piclidenoson in another Phase III trial for treating patients suffering from moderate-to-severe psoriasis.






