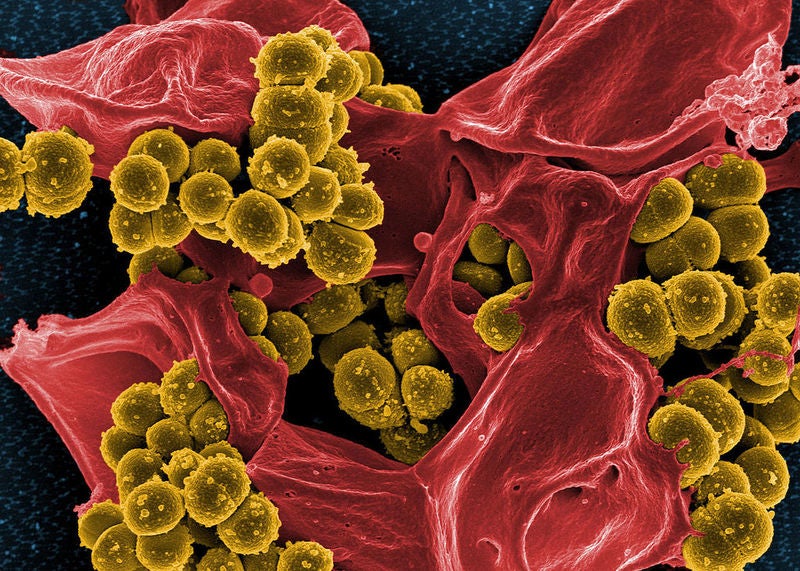
Debiopharm International has initiated a Phase II clinical trial of its antibiotic afabicin (Debio 1450) to treat staphylococcal infections at bones and joints.
Afabicin is a potent, Staphylococcus-selective antibiotic designed to inhibit FabI. It comes in oral and intravenous (IV) formulations.
The therapeutic is also said to be against staphylococci strains resistant to the existing standard-of-care antibiotics such as beta- lactams, vancomycin, daptomycin or linezolid.
The safety, tolerability and efficacy of afabicin IV/oral will be assessed in 60 patients at clinical sites across the US and Ukraine as part of the randomised, open-label active-controlled trial.
Debiopharm International medical director Mahdi Farhan said: “This trial is a key step forward for afabicin clinical development, but even more for patients suffering from severe bone and joint infections who are left with too few treatment options.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData“Remember that afabicin is a first in class selective Staphylococcus aureus antibiotic and that Staphylococcus aureus is responsible for most of the BJI infections.
“So specifically targeting the responsible bug is not only providing new ammunition against a well-known high priority pathogen, but it is also embracing latest stewardship program and treatment trends that all point to a better use of antibiotics.”
The Phase II trial’s primary endpoints are safety and tolerability, which will be tracked as the number of subjects with adverse events (AEs) and serious adverse events (SAEs).
It will also monitor secondary endpoints such as the number of responders, resolution of disease-specific signs and symptoms, improvement of inflammation and microbiological eradication of the baseline pathogen.
Debiopharm expects to complete patient enrolment for the trial by mid-2020.







