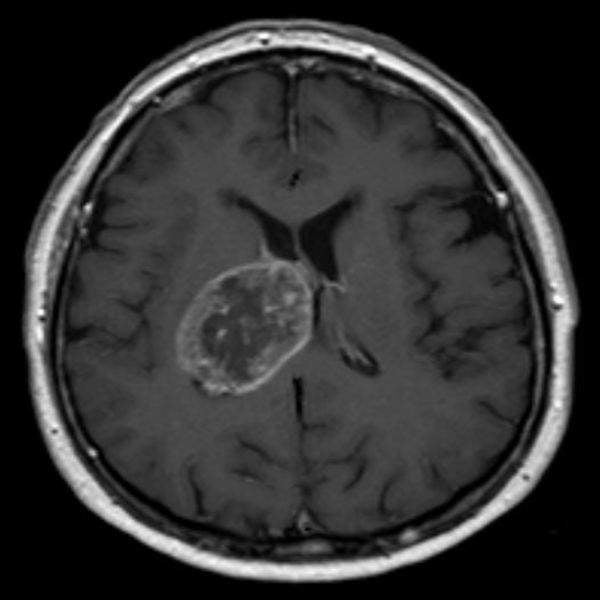
US-based Diffusion Pharmaceuticals has started dosing patients in the Phase III INTACT clinical trial of its product candidate trans sodium crocetinate (TSC) for the treatment of newly diagnosed inoperable glioblastoma multiforme (GBM).
TSC is being developed to counteract tumour hypoxia to make treatment-resistant cancer cells more susceptible to the standard radiation therapy (RT) and chemotherapy.
The open-label, randomised, controlled Phase III trial will compare the overall survival of patients receiving a combination of TSC with standard of care (SoC) RT and those having chemotherapy or SOC alone.
Using temozolomide as the SoC drug, the trial will be conducted in approximately 264 subjects at up to 100 sites in the US and Europe.
The INTACT trial will evaluate safety, tolerability and pharmacokinetics (PK) of TSC at doses higher than 0.25mg/kg with temozolomide during combination therapy.
A baseline assessment will be carried out at ten weeks to establish progression-free and survival overall response rate, while ruling out pseudo-progression.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataDiffusion Pharmaceuticals CEO David Kalergis said: “We are pleased to be dosing patients so soon following the opening of the INTACT trial just a few weeks ago.
“We believe that this Phase III study will offer new hope for inoperable GBM patients who are administered TSC along with their standard therapies.”
In a prior Phase II trial, inoperable GBM patient subgroup is reported to have demonstrated increased survival with the addition of TSC to the treatment regimen, when compared with historical controls.