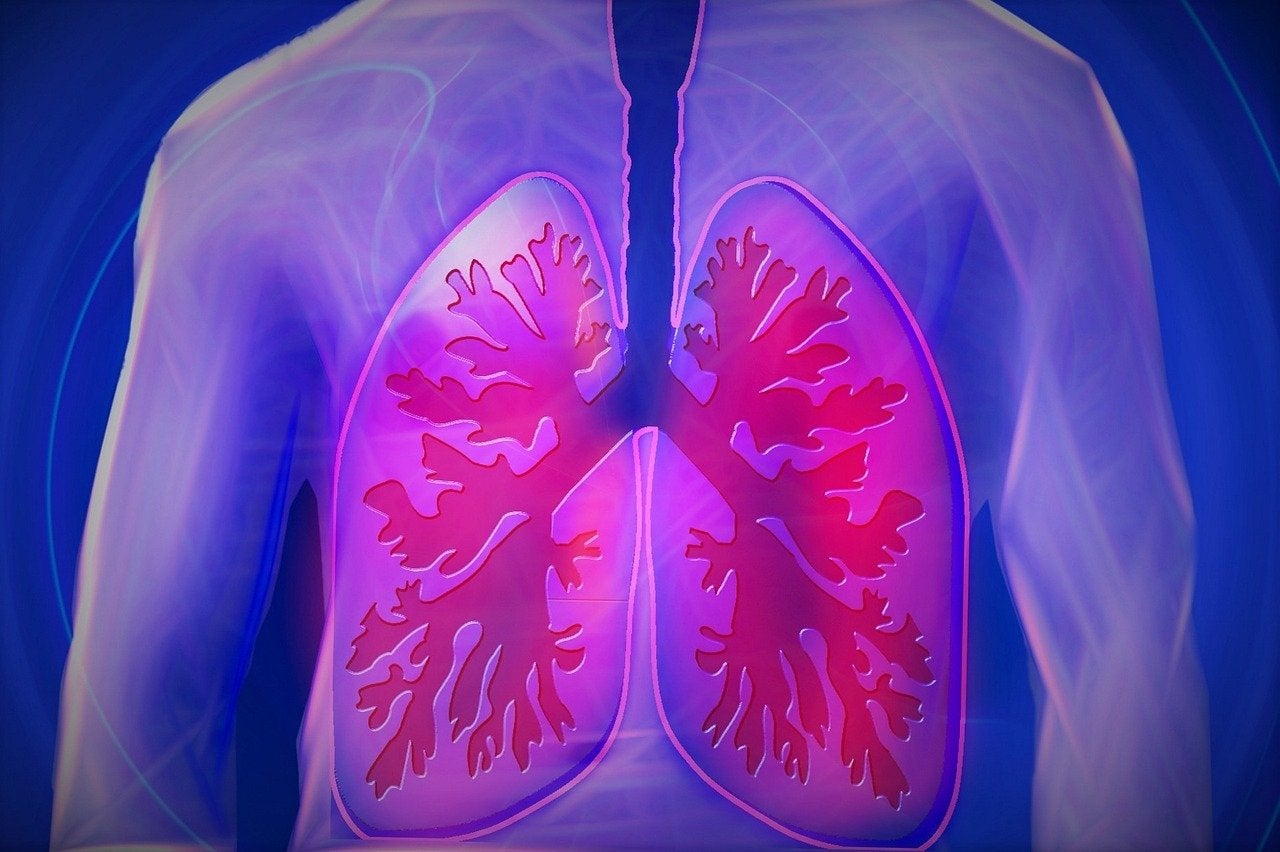
Galapagos and Gilead Sciences have announced the decision to discontinue the ISABELA Phase III clinical studies of ziritaxestat (GLPG1690) in patients with idiopathic pulmonary fibrosis (IPF).
Discovered by Galapagos, ziritaxestat is an investigational autotaxin inhibitor. In July 2019, Gilead in-licensed ex-European rights to ziritaxestat and began sharing the Phase III development costs.
The latest move is based on the recommendation by the Independent Data Monitoring Committee (IDMC). Following a regular review of unblinded data, the committee noted that ziritaxestat’s benefit-risk profile no longer supported the continuation of these studies.
Galapagos chief medical officer Dr Walid Abi-Saab said: “We are very disappointed not to be able to bring a novel medication to patients suffering from such a devastating disease with high unmet need.
“We intend to learn from this data in our continued commitment to developing therapies in IPF and fibrosis.”
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataInvestigators are being notified of the discontinuation of the studies and the participants will be informed to stop the investigational treatment.
The ISABELA Phase III programme has two trials that have been identically designed: ISABELA 1 & 2, intending to enrol a total of 1,500 IPF patients.
Patients continued on their standard of care background treatment and randomly received either a once-daily dose of ziritaxestat 200mg or 600mg or placebo.
The rate of decline of forced vital capacity until week 52 was the primary endpoint.
All clinical trials with ziritaxestat, including the long-term extension of the Phase IIa NOVESA trial in systemic sclerosis, will be discontinued.
Last May, Gilead Sciences and Galapagos reported positive top-line results from the Phase IIb/III SELECTION clinical trial of filgotinib for the treatment of moderately to severely active ulcerative colitis (UC).








