
French biotechnology company Abivax has begun patient enrolment in the ABX464-005 clinical trial of ABX464 for the treatment of HIV.
A part of the firm's anti-viral platform, ABX464 is an oral, first-in-class, small-molecule drug candidate expected to be a functional cure for HIV patients.
The ABX464-005 trial is designed to evaluate the effect and pharmacokinetics of ABX464 in HIV cellular reservoirs of 24 patients and 12 healthy subjects.
An ongoing Phase IIa trial ABX464-004 is focusing on blood reservoirs, while the ABX464-005 trial will involve gut reservoirs.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataSee Also:
The placebo-controlled ABX464-004 trial is being conducted in Europe to investigate the effects in monocytes and T-cells with integrated viral DNA.
Abivax chief medical officer Dr Jean-Marc Steens said: "Our understanding of this potential functional cure for HIV will benefit from this trial, which can demonstrate the prevention of viral replication originating from the HIV reservoir.
“The ability of ABX464, in contrast to existing drugs, to act on already infected immune cells like macrophages residing in the gut, will be further explored through specific ex-vivo analyses of these reservoir cells removed from patients in regular biopsies during the trial.”
The ABX464-005 trial is to be performed at the Germans Trias i Pujol University Hospital Badalona, Spain, and will involve administration of ABX464 to the patients already receiving anti-retroviral treatment.
Rectal biopsies will be analysed at certain intervals to quantify the viral load and the level of inflammation at the reservoir.
It is anticipated that the trial would allow better understanding of ABX464's biological mechanism associated with the long-term efficacy on the viral load rebound.
The initial results from the ABX464-005 trial are expected to be reported in the third quarter of this year.
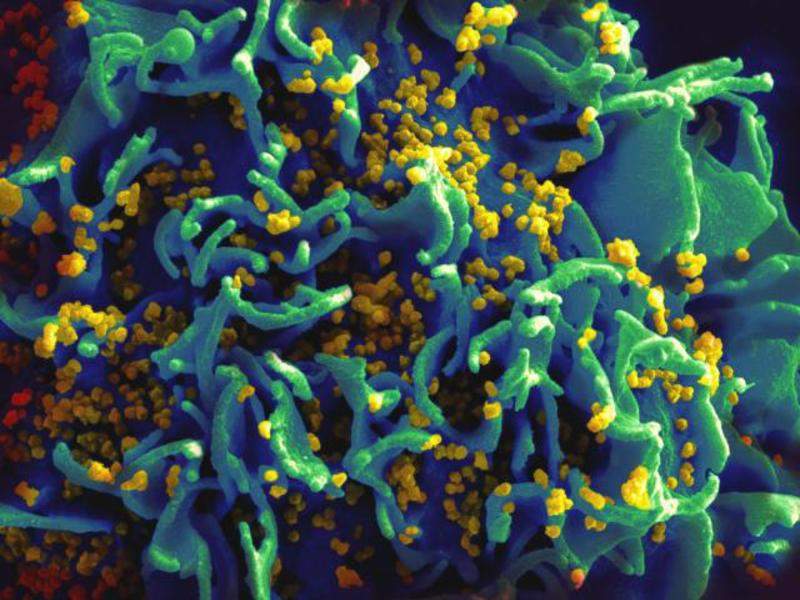
Image: Scanning electromicrograph of an HIV-infected T-cell. Photo: courtesy of NIAID.







