
Swiss-based pharmaceutical firm Asceneuron is planning to initiate a Phase I clinical trial of ASN120290 in healthy volunteers, following the regulatory approval.
Part of the class of O-GlcNAcase enzyme inhibitors, ASN120290 is an oral tau inhibitor that targets toxic neurofibrillary tau tangle accumulation to counter neurodegenerative diseases such as dementia.
Neurofibrillary tangles are reported to be the primary cause for neurodegeneration and clinical symptoms.
The randomised, double-blind, placebo-controlled Phase I trial is designed to evaluate the safety, tolerability, food effect, pharmacokinetics and pharmacodynamics of single and multiple doses of the product.
See Also:
Asceneuron chief executive officer and co-founder Dirk Beher said: “This is a significant milestone for Asceneuron and marks our transition to a clinical stage company.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData"It is also a major achievement for our scientific team as ASN120290 is our first in-house developed molecule reaching the clinic and was designed to easily enter the brain.
"New approaches to treat dementia are urgently required and preventing toxic tau tangle formation with our O-GlcNAcase inhibitor represents a new mechanism of action.”
The optimal dose selection of ASN120290 for future studies will be supported by the assessment of a blood-based biomarker in the trial.
Next year, the firm plans to further investigate ASN120290 in a Phase II proof-of-concept trial for the treatment of the orphan tauopathy disease progressive supranuclear palsy (PSP), after the successful completion of Phase I study.
Preclinical studies indicated that ASN120290 can minimise the accumulation of the tau protein toxic aggregates into neurofibrillary tangles.
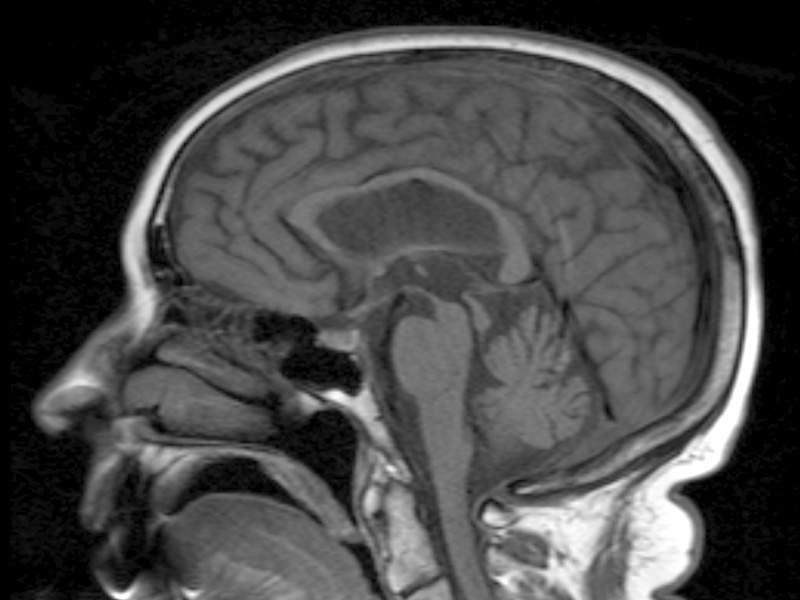
Image: CT illustrating progressive supranuclear palsy. Photo: courtesy of Dr Laughlin Dawes/Wikipedia.