
French company Genkyotex has started patient enrolment in a Phase II clinical trial of GKT831 for the treatment of patients with primary biliary cholangitis (PBC).
GKT831 is an inhibitor of the enzymes NOX1 and NOX4.
While the subject recruitment is initiated at a centre in the US, the firm intends to activate more than 50 clinical sites across several European countries, as well as in Canada and the US.
The 24-week, double-blind, placebo controlled, multi-centre Phase II trial is designed to assess the safety and efficacy of GKT831 in patients experiencing inadequate response to ursodeoxycholic acid.
Approximately 102 subjects are set to be enrolled and administered with either 400mg once-daily or 400mg twice-daily GKT831.
See Also:
Genkyotex executive vice-president and chief medical officer Philippe Wiesel said: "Alleviating progressive liver injury and fibrosis is an important therapeutic objective in patients with active PBC.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData"We are grateful to the global network of experienced investigators who will participate in this important Phase II clinical study. We are now focused on delivering trial results over the next 18 months."
The primary objective of the trial is demonstration of therapeutic activity through a decrease in gamma glutamyl transpeptidade, while the secondary efficacy endpoints are markers of liver inflammation and injury, and non-invasive markers of liver fibrosis.
The trial will further assess GKT831’s clinical safety profile, pharmacokinetics and effect on bile acid metabolism, itching, autoimmunity and quality of life.
The interim top-line results from the trial will be available in the first half and the complete results in the second half of next year.
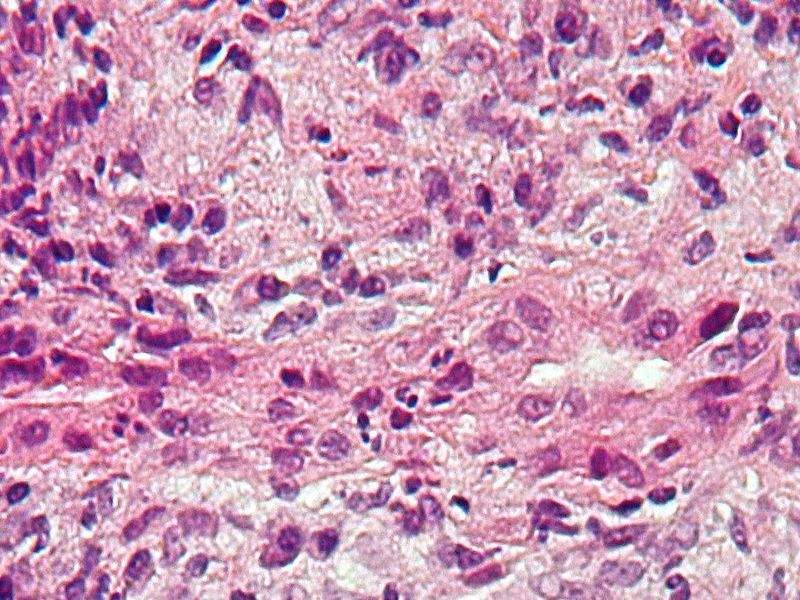
Image: Micrograph of PBC. Photo: courtesy of Nephron.