
Clinical-stage biotechnology firm GeoVax Labs has initiated the next human Phase I clinical trial (HVTN 114) of its GOVX-B11 vaccine developed to prevent HIV.
With DNA and modified vaccinia Ankara (MVA) vaccine components, GOVX-B11 is designed for use against the clade B subtype of HIV.
The recombinant DNA vaccine is intended to prime a immune response, while the recombinant MVA vaccine will bolster the response. Both the vaccine components generate non-infectious, virus-like particles in the cells of the vaccinated person.
Approximately 100 patients who participated in the HVTN 205 Phase IIa trial of the GOVX-B11 vaccine will be enrolled in HVTN 114.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataSee Also:
The ability of late boosts vaccinations comprised of GeoVax MVA62B vaccine with or without a gp120 protein vaccine to enhance the antibody responses primed by the vaccine will be tested in the Phase I trial.
GeoVax HIV vaccine programme director Harriet Robinson said: "Information from this trial will contribute to the design of human clinical trials testing our vaccine in the presence and absence of the gp120 proteins that are currently being prepared for use with GOVX-B11.”
The Phase I trail is being carried out by the HIV Vaccine Trials Network (HVTN) and is funded by the National Institute of Allergy and Infectious Diseases (NIAID).
The HVTN has also reported positive reports for its trials of GOVX-B11 conducted using multiple doses and regimens in around 500 individuals.
In August last year, NIAID rendered GeoVax with a $200,000 staged vaccine development contract with additional development options of up to $7.6m to manufacture the DNA component of the vaccine for use in future clinical trials.
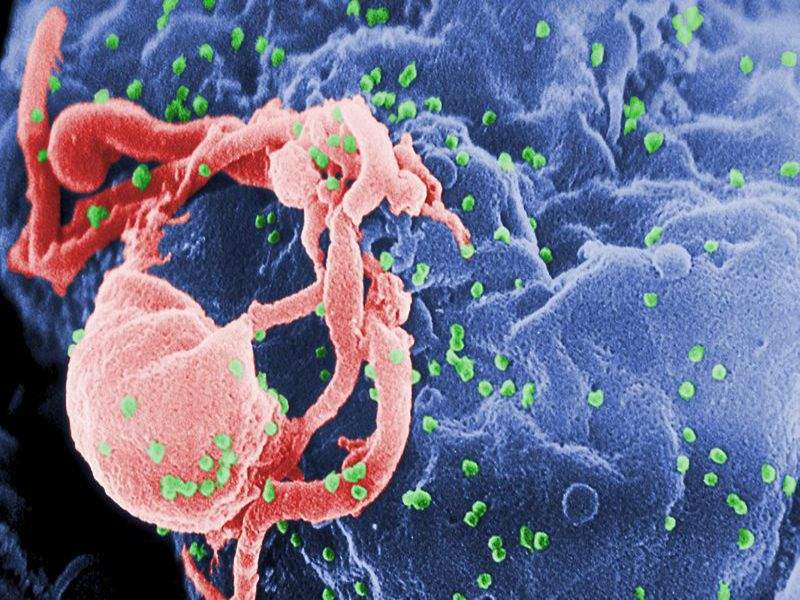
Image: Scanning electron micrograph of HIV-1 budding. Photo: courtesy of C. Goldsmith.