
US-based biotechnology company Imara has initiated its Phase I clinical study of IMR-687 to treat patients with sickle-cell disease and other haemoglobinopathies.
IMR-687 is an orally administered, highly potent candidate developed to selectively inhibit phosphodiesterase-9 (PDE9i) in blood cells.
The inhibition of PDE9 reduces white blood cell stickiness that prevents blockage of blood vessels.
Sickle-cell disease is a rarely occurring, genetically inherited condition that alters haemoglobin, which is responsible for transporting oxygen throughout the body.
See Also:
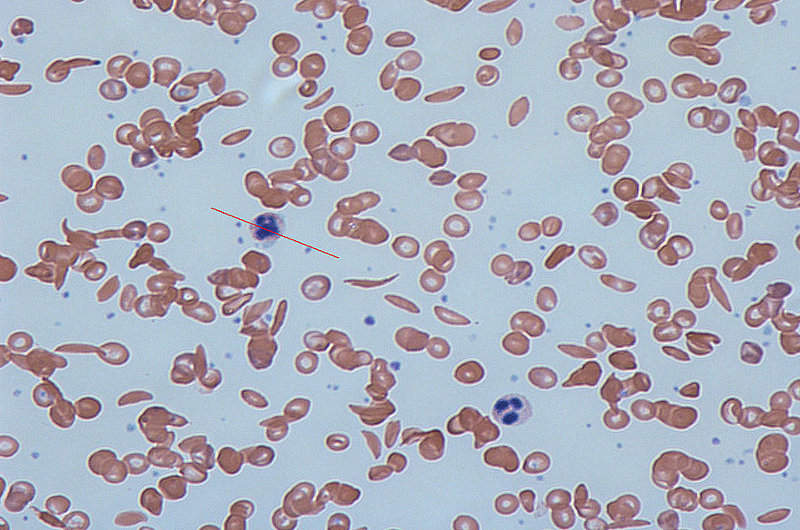
Altered haemoglobin distorts red blood cells into a stiff and inflexible sickle, or crescent, which gets stuck in small blood vessels.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataThe condition prevents blood carrying oxygen from reaching tissues and organs, thereby leading to vaso-occlusive crisis (VOC), acute chest syndrome (ACS), and permanent damage to organs including the liver, spleen, kidney and brain.
The Phase I clinical study is being conducted to assess the safety and tolerability of Imara’s lead product candidate, IMR-687.
Imara founder, president and CEO James McArthur said: “More than 160,000 individuals are living with sickle-cell disease in the United States and Europe with many more in Africa and Asia, yet, there remains a serious medical need facing those whose lives are burdened by this devastating disease.
“Initiating this first study in humans is a critically important milestone as we work to advance new therapies for people living with sickle-cell disease.”
Preclinical studies have suggested the efficacy of IMR-687 in reducing both the sickling of red blood cells and blood vessel occlusion.
Image: Human blood with normal and sickle-shaped cells. Photo: courtesy of Dr Graham Beards.