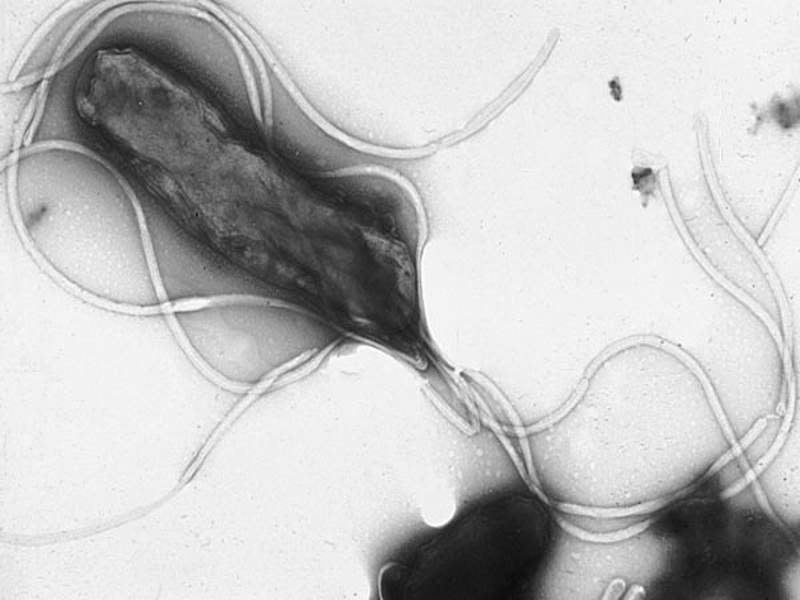

Israeli-based specialist biopharmaceutical firm RedHill Biopharma has commenced a confirmatory Phase III ERADICATE Hp 2 clinical trial of RHB-105 (Talicia) for the treatment of patients with Helicobacter pylori (H. pylori) infection.
Talicia is an oral, fixed-dose combination of two antibiotics and a proton pump inhibitor (PPI) in a single capsule.
The two-arm, randomised, double-blind, active comparator, confirmatory Phase III trial is designed to recruit 444 subjects at approximately 65 clinical centres in the US.
The trial will compare the product with a combination therapy regimen of amoxicillin and omeprazole at equivalent doses in non-investigated dyspepsia patients with a confirmed infection.
See Also:
Patients will be administered four capsules three times a day of either Talicia or the active comparator over 14 days.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataThe trial’s primary endpoint is eradication of the infection at 42 through to 70 days after the start of treatment.
The previous Phase III ERADICATE Hp trial of the product is reported to have demonstrated 89.4% efficacy in eradicating the infection when compared to standard-of-care (SoC) treatment.
The firm expects that the potential favourable results from the ERADICATE Hp 2 trial, positive results from the ERADICATE Hp trial and findings from the supportive PK programme will support a US new drug application (NDA) for Talicia.
The US Food and Drug Administration (FDA) has granted the product with qualifying infectious disease product (QIDP) designation, a fast-track development pathway, NDA priority review status and extended market exclusivity in the country for eight years.
Image: Electron micrograph of H. pylori. Photo: courtesy of Yutaka Tsutsumi/Professor department of pathology / Fujita Health University School of Medicine.







