
Sanofi Genzyme has begun enrolment and dosed the first UK patient for its pivotal Phase III COMET clinical trial of neoGAA to treat Pompe disease.
NeoGAA is an investigational, second-generation, alglucosidase alfa enzyme replacement therapy designed for enhanced receptor targeting and enzyme uptake with greater affinity for the M6P receptors on muscle cells.
It is aimed at improving glycogen clearance and improving on the clinical efficacy achieved with alglucosidase alfa.
Pompe disease is a progressive, debilitating and often fatal neuromuscular disease triggered by a genetic deficiency or dysfunction of the lysosomal enzyme acid alpha-glucosidase (GAA).
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataNewcastle Hospitals NHS Foundation Trust and Newcastle University John Walton Muscular Dystrophy Research Centre principal investigator Professor Volker Straub said: “Pompe disease is a serious, progressive and debilitating disorder.
“Dosing the first patient in the UK is an exciting and important milestone in our quest to advance treatment options for patients with this disease.”
The Phase III multi-centre, multi-national, double-blinded COMET study is being conducted to compare the safety and efficacy of repeated bi-weekly infusions of neoGAA and alglucosidase alfa in 96 treatment-naïve patients with late-onset Pompe disease.
The study is primarily focused on determining the effect of neoGAA on respiratory muscle strength as measured by percent predicted forced vital capacity in the upright position.
It will also assess functional endurance measured by the six minute walk test, muscle strength, motor function, health-related quality of life, and patient reported outcomes.
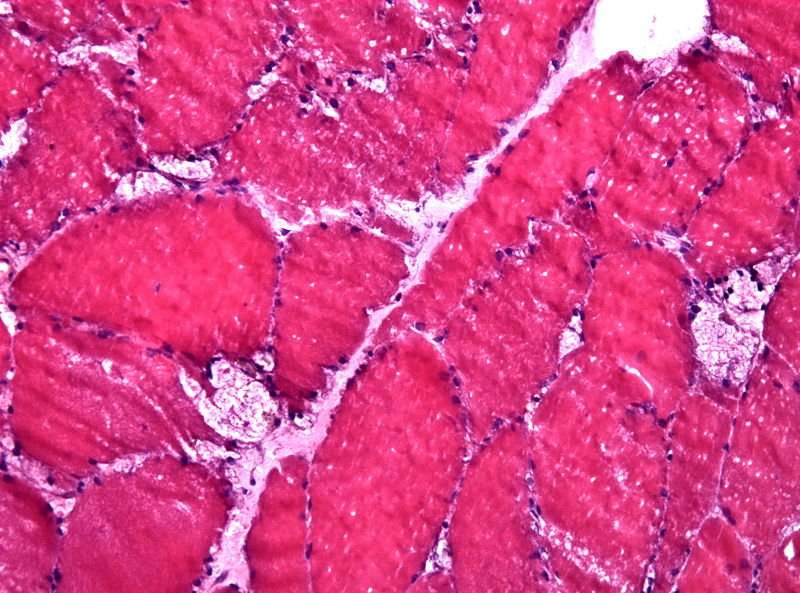
Image: Muscle biopsy reveals large vacuoles in a case of Pompe disease. Photo: courtesy of Jensflorian.