
University College London (UCL) has carried out a study that indicated the potential of a common diabetes drug called exenatide as a disease-modifying therapy for treating Parkinson’s disease.
Funded by the Michael J Fox Foundation for Parkinson’s Research (MJFF), the study involved administration of 60 Parkinson’s disease patients with exenatide once every week over one year.
The patients were monitored by the researchers at the National Hospital for Neurology and Neurosurgery (NHNN).
Subjects who self-injected exenatide were found to perform better in movement (motor) tests, when compared to those administered with placebo.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataSee Also:
UCL Institute of Neurology professor Tom Foltynie said: “This is a very promising finding, as the drug holds potential to affect the course of the disease itself, and not merely the symptoms.
“With existing treatments, we can relieve most of the symptoms for some years, but the disease continues to worsen.”
While exenatide patient group demonstrated better motor function at 48 weeks with persistent effect at the 12-week follow-up, placebo group indicated decreased motor scores at the 48-week and the following 12-week tests.
On a 132-point scale of measures such as tremors, agility and speech, the advantage of four points was observed to be statistically significant.
The researchers intend to expand the evaluation of exenatide into a long-term study as the current one failed to conclusively indicate any disease modification or noticeable symptom-related improvements.
Developed to activate GLP-1 hormone receptors in the pancreas for stimulation of insulin production, exenatide has been in use to treat type 2 diabetes since 2005.
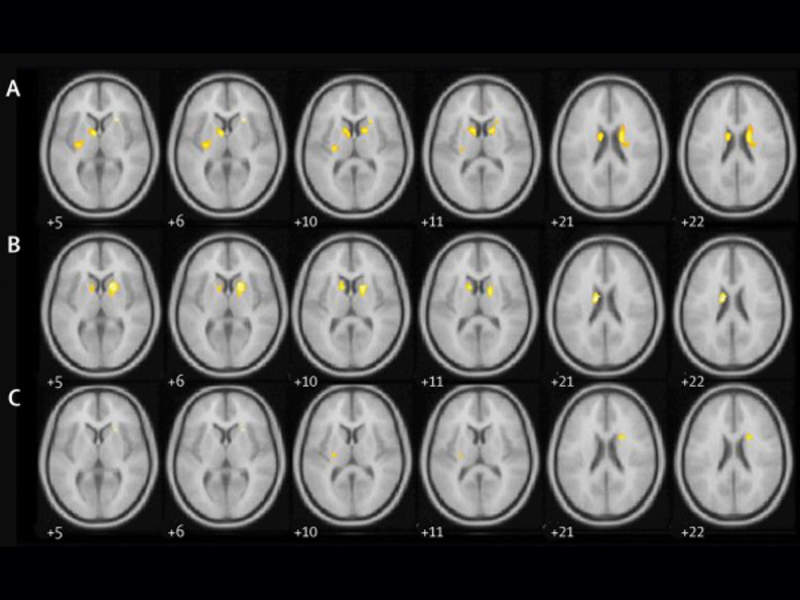
Image: Brain imaging of study participants. Photo: courtesy of University College London.