Researchers at the University of Nottingham and Nottingham University Hospitals NHS Trust, UK, have collaborated with a team from the Cleveland Clinic in Ohio, US, for a clinical trial for the treatment of multiple sclerosis (MS).
The $10.6m international five-year trial will evaluate early intensive and escalation disease-modifying treatment (DMT) approaches for the disease.
Funded by the Patient-Centred Outcomes Research Institute in the US, the trial will be performed in 800 relapsing-remitting multiple sclerosis (RRMS) patients, 400 each in the US and the UK.
University of Nottingham associate professor Dr Nikos Evangelou said: “This is a joint effort between neurologists, patients with MS and MS societies in the UK and US coming together to tackle the most important question in MS treatment – how intensive should the initial treatment of MS be?
“Currently, across the UK, the variability of treatments used in different centres is staggering. This is a reflection of the fact that little is known about how best to treat it and this is what we are attempting to tackle.”
The multi-centre randomised trial will compare the safety, efficacy and tolerability of early treatment using high-efficacy medicines and therapy after disease progression.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataThe ability of these approaches to slow brain volume loss will be determined by measuring patient functioning in various areas such as cognition, arm and leg function, and eyesight.
The trial will also monitor the impact of treatment on MS symptoms and quality of life, along with any potential side effects.
Findings from this trial are expected to aid neurologists in making overall treatment choices.
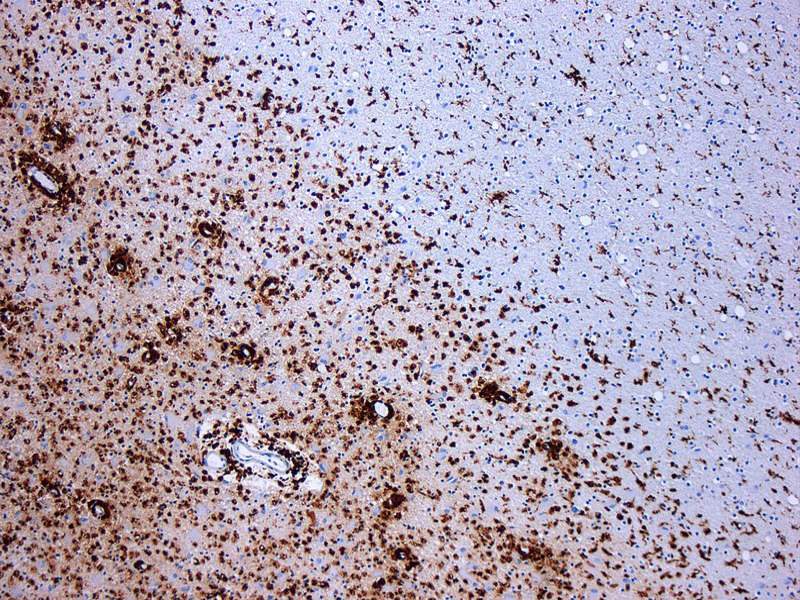
Image: Photomicrograph of a demyelinating MS-lesion. Photo: courtesy of Marvin 101.