Nicox has commenced a Phase ll clinical trial to examine the efficacy and safety of NCX 470 in comparison with latanoprost 0.005% for the treatment of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
The multi-centre, double-masked, 28-day, parallel group dose-ranging trial has already enrolled ten patients.
During the trial, 420 patients are expected to randomise in various clinical sites across the US.
The trial’s primary endpoint is the mean reduction in diurnal IOP after four weeks of treatment, and its overall objective is to identify the appropriate dose of NCX 470 for Phase lll studies of the drug.
Top-line results from the trial are scheduled to be available by the second half of next year.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataNicox chairman and CEO Michele Garufi said: “Based on our preclinical data, we believe that NCX 470 may provide a clinically meaningful improvement over the current standard of care, and become a first-line therapy for these patients.
“The development of NCX 470 is testimony to the power of our efforts fuelled by the seamless collaboration between our US and European R&D teams, and follows on the back of the Food and Drug Administration (FDA) approval of our first-generation NO-donating compound VYZULTA, commercialised in the US since last December by our partner Bausch + Lomb.”
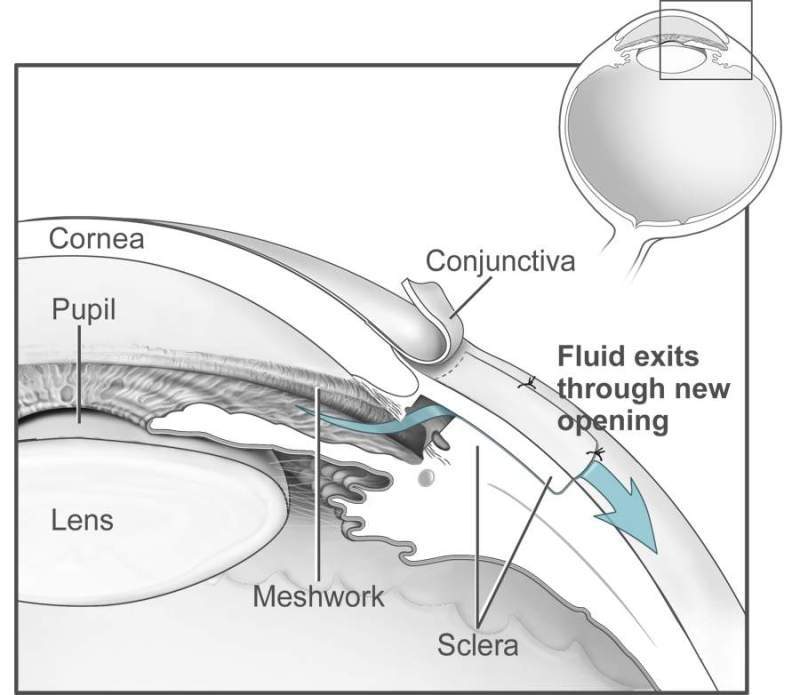
NCX 470 is a novel chemical entity developed as an ophthalmic solution of the new, second-generation nitric oxide (NO)-donating prostaglandin analogue for reducing IOP in patients with open-angle glaucoma and ocular hypertension.
Glaucoma is a group of ocular diseases which damages the optic nerve, thereby leading to peripheral and, ultimately, central visual field loss.








