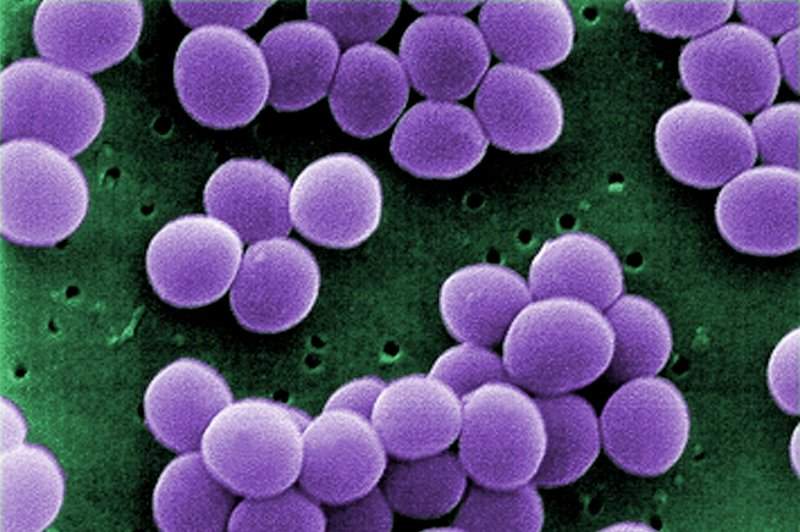
NovaDigm Therapeutics has initiated a Phase IIa trial analysing NDV-3A for the treatment of Staphylococcus aureus (S. aureus) colonisation in a high-risk population of military trainees.
The placebo controlled trial aims to evaluate the safety, immunogenicity and efficacy of NDV-3A in reducing nasal/oral acquisition of S. aureus.
The trial is being carried out at the Uniformed Services University of the Health Sciences (USU) and is currently enrolling US Army Infantry trainees at Fort Benning, Georgia.
Around 400 subjects, including infantry trainees from four classes, are expected to be enrolled in the trial.
During the trial, subjects will receive NDV-3A shortly after their arrival at Fort Benning. It will also involve follow-up visits throughout the 14-week training cycle to examine S. aureus colonisation status of the subjects.
NovaDigm Therapeutics CEO Timothy Cooke said: “Military recruits who are colonised with Staph aureus are at increased risk for skin infections, which can manifest in various ways including benign boils, cellulitis or more severe, invasive soft-tissue infections.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData“The goal of this study is to reduce colonisation during basic training with the NDV-3A vaccine.
“Following on the results of this trial, a larger study would be conducted to demonstrate a reduction in skin infections.”
NDV-3A is currently under development to serve as an immunotherapy and preventative vaccine for infections caused by several species of the fungus Candida. These include Candida albicans, multidrug-resistant Candida auris and the bacterium Staphylococcus aureus, including methicillin resistant S. aureus (MRSA).






