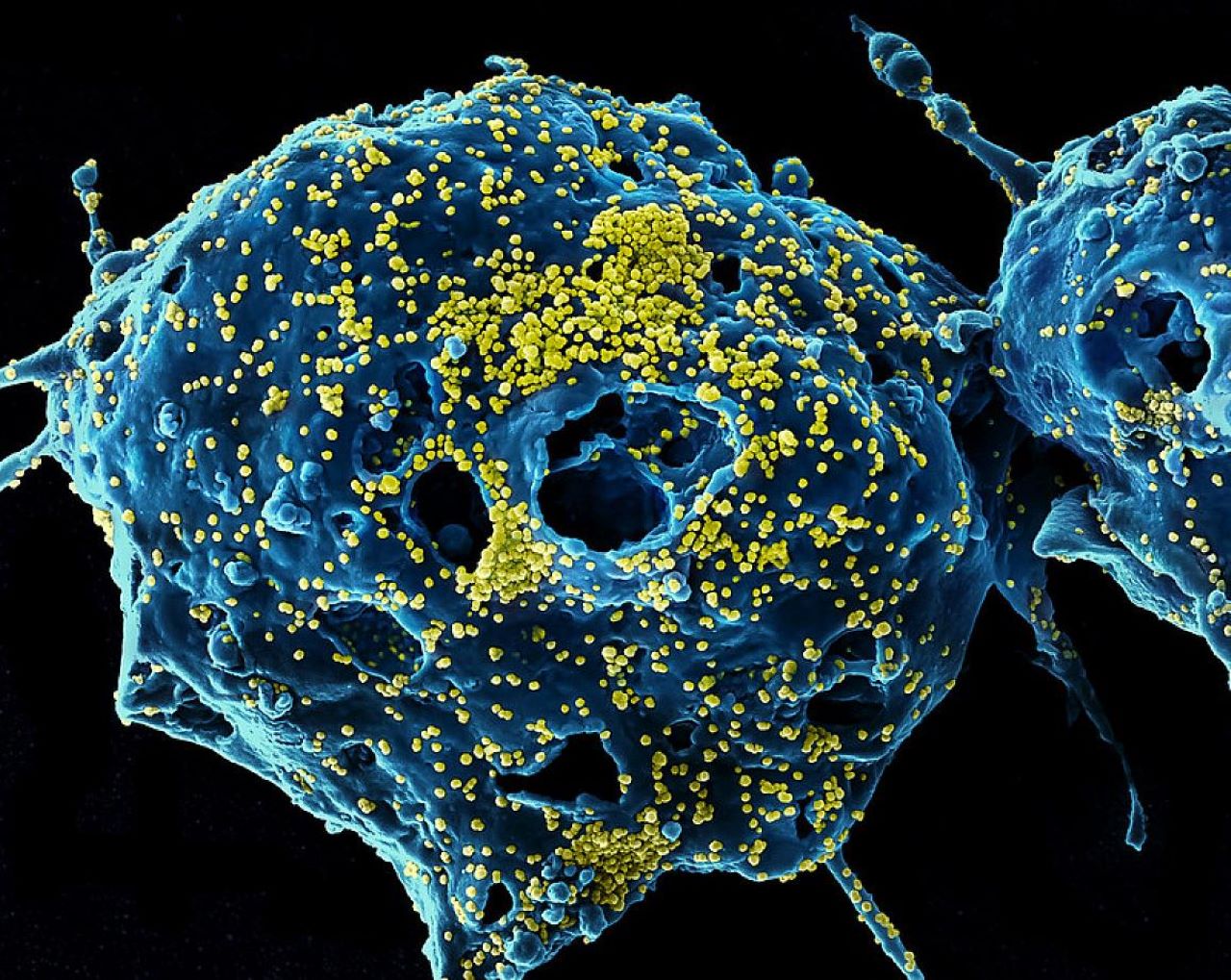
The US National Institutes of Health (NIH) has reported that the Phase I clinical trial of Regeneron’s two monoclonal antibodies (mAbs) directed against the Middle East respiratory syndrome coronavirus (MERS-CoV) was shown to be well tolerated and safe when administered simultaneously to healthy adults.
The investigational mAbs, REGN3048 and REGN3051 are discovered and developed by Regeneron and target the MERS-CoV spike protein used by the virus to bind to and infect cells.
In 2012, the first recognised case of MERS was reported in Jordan.
Sponsored by the NIH unit National Institute of Allergy and Infectious Diseases (NIAID), the randomised, placebo-controlled trial is the first to analyse experimental antibodies in humans.
It was carried out at a clinical trial site in California, WCCT Global, enrolling 48 healthy adult participants, with 36 receiving mAbs.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataThey were given an intravenous infusion of mAbs or placebo and followed up for 121 days.
The NIH noted that no serious adverse events were observed in the antibody trial.
Researchers at Regeneron and the University of Maryland, College Park gave REGN3048 and REGN3051 sequentially and in combination to genetically modified mice that can be infected with MERS-CoV, in preclinical studies.
Data showed that both the mAbs, on administration one day before coronavirus exposure, lowered virus levels later detected in the lungs, while co-administration was more effective in providing protective effects than either of the mAb alone.
Furthermore, co-administering the mAbs a day after MERS-CoV exposure reduced viral levels and lowered tissue damage in the lungs of the mice versus mice receiving placebo.
The latest findings and the preclinical data indicate the potential efficacy and use of monoclonal antibody therapy for preventing or treating MERS-CoV. It also sets the basis for developing spike-targeted mAb therapies for other infectious diseases such as SARS-CoV-2.







