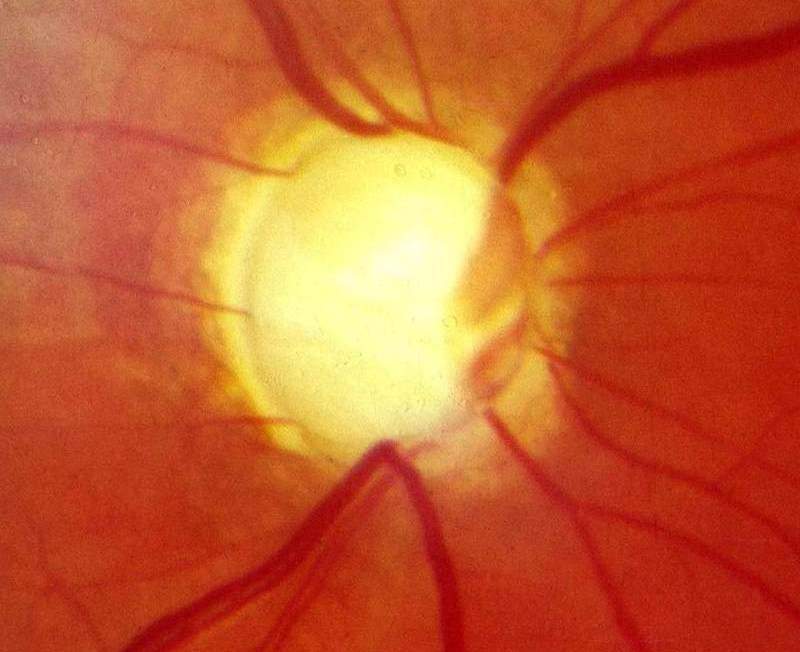
Santen has started the Phase III SPECTRUM trial of omidenepag isopropyl (DE-117) 0.002% against timolol maleate 0.5% for the treatment of patients with glaucoma or ocular hypertension (OHT).
The SPECTRUM programme includes two pivotal, active-controlled trials to examine the efficacy and safety of omidenepag isopropyl in treating patients with OHT.
Each of the SPECTRUM 3 and SPECTRUM 4 trials will include around 400 adult patients and up to 30 paediatric patients with glaucoma or OHT across 70 clinical sites in the US.
Patients will be randomised in a 1:1 ratio to receive either omidenepag isopropyl once-daily or a twice daily dose of timolol.
SPECTRUM 3 is a 12-month trial that features a three-month double-masked treatment period and a nine-month open-label period, while SPECTRUM 4 will be conducted for a period of three months.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataThe primary objective of both trials is to examine whether once-daily omidenepag isopropyl is as effective as twice-daily timolol in lowering intraocular pressure (IOP) after three months.
Santen chief scientific officer and global research and development head Naveed Shams said: “Santen’s deep commitment to glaucoma drives us to advance therapeutic innovation in areas of unmet needs.
“Currently when glaucoma treatments fail, patients urgently need therapeutic options with differentiated mechanisms of action to effectively reduce intraocular pressure.
“By increasing the aqueous humour outflow through both uveoscleral and trabecular pathways, omidenepag isopropyl has the potential to provide physicians with a novel option to treat elevated pressure in glaucoma.”
Commencement of the Phase III SPECTRUM programme follows positive Phase I/II, II and IIb trials that demonstrated omidenepag isopropyl 0.002% as being the most appropriate dose, working similarly to latanoprost in reducing IOP.
Omidenepag isopropyl was also found to be generally safe and well tolerated.
Common side-effects of the drug, including iris and eyelid pigmentation, abnormal eyelash changes and deepening of upper eyelid sulcus, were not observed in a long-term use study.







