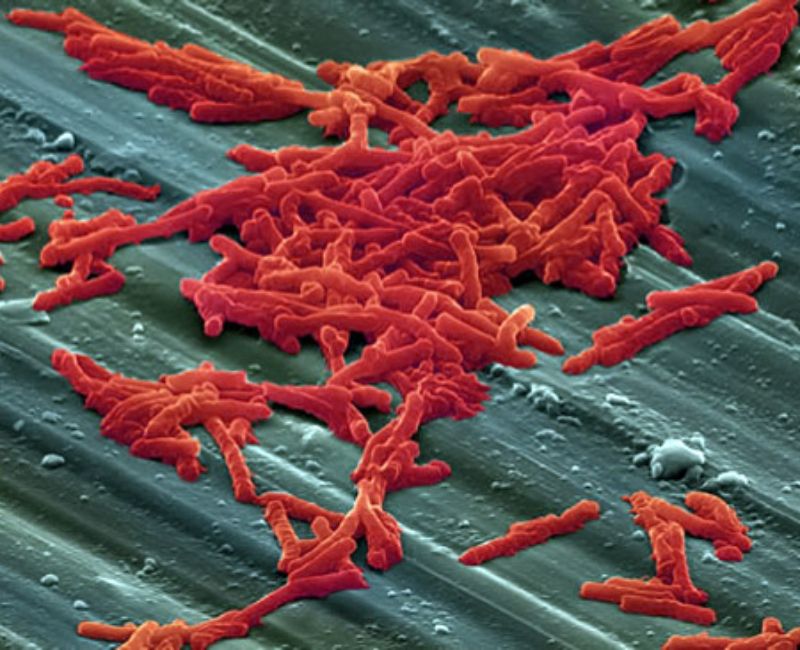
Seres Therapeutics has reported positive top-line data from the Phase III ECOSPOR III clinical trial of SER-109 to treat recurrent C difficile infection (CDI).
The study met the primary endpoint, demonstrating a statistically significant absolute reduction of 30.2% in the proportion of patients who had a recurrence in CDI within eight weeks of SER-109 administration compared to placebo.
Data showed that 11.1% of participants on SER-109 experienced a CDI recurrence versus 41.3% of those on placebo.
The company expects the efficacy data to support a biologics licence application (BLA) to the US Food and Drug Administration (FDA).
The safety data from the trial was favourable, where the adverse event profile was comparable to that of placebo.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataSeres Therapeutics president and CEO Eric Shaff said: “Based on our prior discussions with the FDA, we believe this trial should provide the efficacy basis for submitting an application for product approval.
“We look forward to meeting with the FDA as soon as possible to discuss the regulatory path forward with the goal of bringing SER-109 to patients as a first-in-class microbiome therapeutic.”
SER-109 is an investigational, oral, biologically-derived microbiome therapeutic intended to decrease CDI recurrence.
The multi-centre, randomised, placebo-controlled ECOSPOR III trial compared the drug candidate to placebo following standard of care antibiotic treatment in a total of 182 patients with multiply recurrent CDI.
The primary efficacy endpoint of the study was the proportion of subjects with recurrent CDI at up to week eight. Participants are analysed for CDI recurrence until 24 weeks post-treatment as a secondary endpoint.
A SER-109 open-label study is being performed at certain clinical sites that were part of the ECOSPOR III trial, with plans to expand the study to additional sites.
Data from the open-label study will contribute to the SER-109 safety database.








