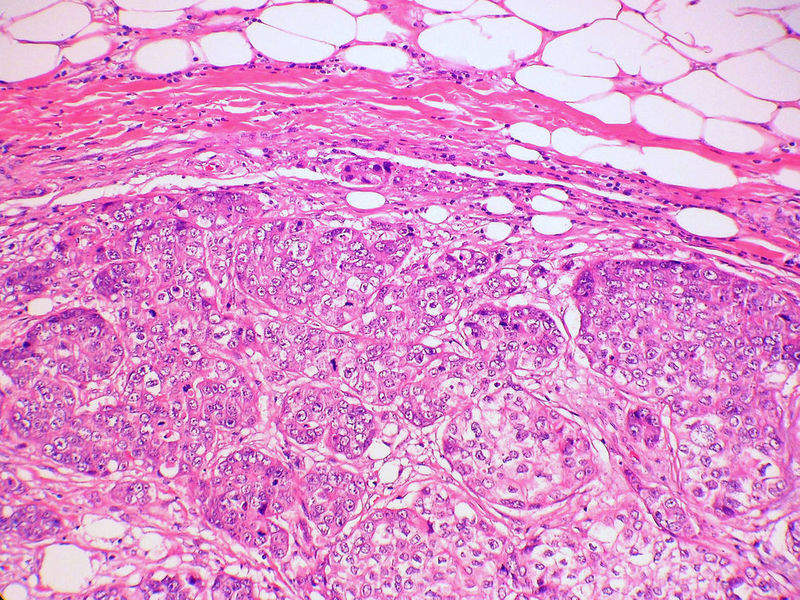
US-based immuno-oncology firm TapImmune has completed patient enrolment in a Phase II clinical trial of its vaccine candidate TPIV200 to treat triple-negative breast cancer (TNBC).
TPIV200 is a peptide-based product being developed to stimulate long-lasting T-cell immune responses against the folate receptor alpha (FRa) molecule found on the surface of many TNBC cancer cells.
The randomised, multi-centre, four-arm trial recruited more than 80 subjects with stage I (T1c) to III TNBC for evaluation of TPIV200 six monthly injections with or without a single treatment of cyclophosphamide before the vaccination.
The trial will establish an optimal vaccine dose and regimen required to maximise the generated immune response.
The primary endpoint of the study is response against FRa.
TapImmune clinical development head Dr Richard Kenney said: “Our Phase II trial focuses on women who have completed initial surgery and radiation / chemotherapy for TNBC at least 60 days prior to randomisation.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData“We believe vaccination with TPIV200 during this important window may potentially delay or prevent cancer recurrence by generating robust T-cell immunity against tumour cells.”
Various dosing strategies assessed during the trial are expected to increase the ability to generate optimal immune responses and prevent disease recurrence in the upcoming trials.
TapImmune expects to report interim immunogenicity results in the first half of next year.
TPIV200 is also being studied in another Phase II trial to investigate disease-free survival in 280 advanced TNBC patients and in a separate Phase II trial for the treatment of ovarian cancer.







