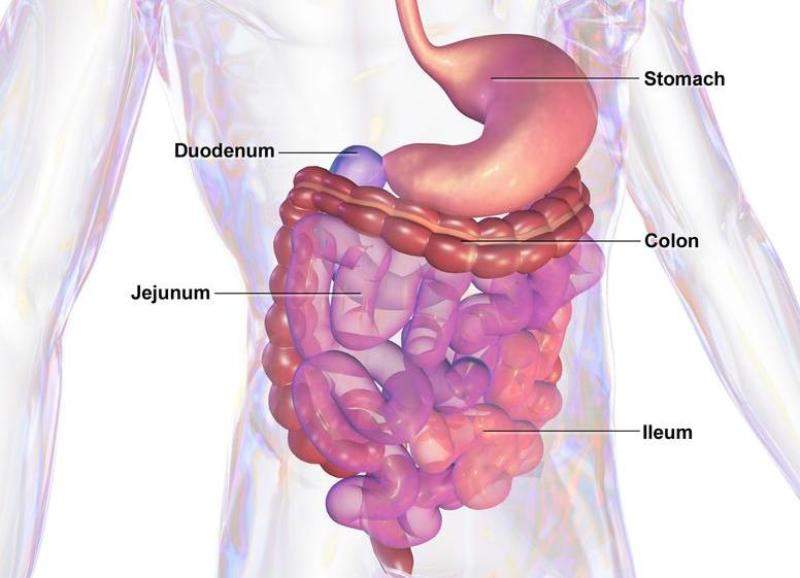
Zealand Pharma has enrolled the first patient in a Phase lll trial investing glepaglutide for the treatment of short bowel syndrome (SBS).
The trial aims to study the efficacy and safety of once and twice-weekly subcutaneous injections of 10mg of glepaglutide in SBS patients who are on parenteral support.
As part of the placebo-controlled, randomised, parallel-group, double-blind, and fixed dose trial, 129 patients will be enrolled at around 40 sites across the US, Canada and Europe.
The trial’s primary endpoint is to confirm the efficacy of glepaglutide in minimising parenteral support volume in SBS patients, while the secondary objectives included evaluation of additional efficacy endpoints, as well as safety and tolerability.
University of Copenhagen Rigshospitalet Department of Gastroenterology professor Palle Bekker Jeppesen is the principal investigator of the trial.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataZealand Pharma executive vice-president and chief medical and development officer Adam Steensberg said: “We are committed to developing glepaglutide as a potentially life-changing treatment for patients with short bowel syndrome, who suffer from reduced or complete loss of intestinal function.”
The US Food and Drug Administration (FDA) previously awarded orphan drug designation to glepaglutide for the treatment of SBS, a chronic and severe condition related to reduced or complete loss of intestinal function.
Glepaglutide is developed as a long-acting, glucagon-like peptide-2 (GLP-2) analog with an effective half-life of around 50 hours.
In an earlier Phase ll trial in patients with SBS, glepaglutide showed promise to increase intestinal absorption after three weeks of treatment.