The US Food and Drug Administration (FDA) has granted clearance for Zucara Therapeutics’ investigational new drug (IND) application for the Phase IIa ZONE clinical trial of ZT-01 to prevent night-time (nocturnal) hypoglycemia in patients with type 1 diabetes (T1D).
The effect of ZT-01 on the frequency of nocturnal hypoglycemia in these patients will be assessed in the multiple-dose, crossover, double-blind, multi-centre, randomised, placebo-controlled study.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Three doses of ZT-01, or a placebo, will be self-administered by the subjects every evening before bed for four weeks.
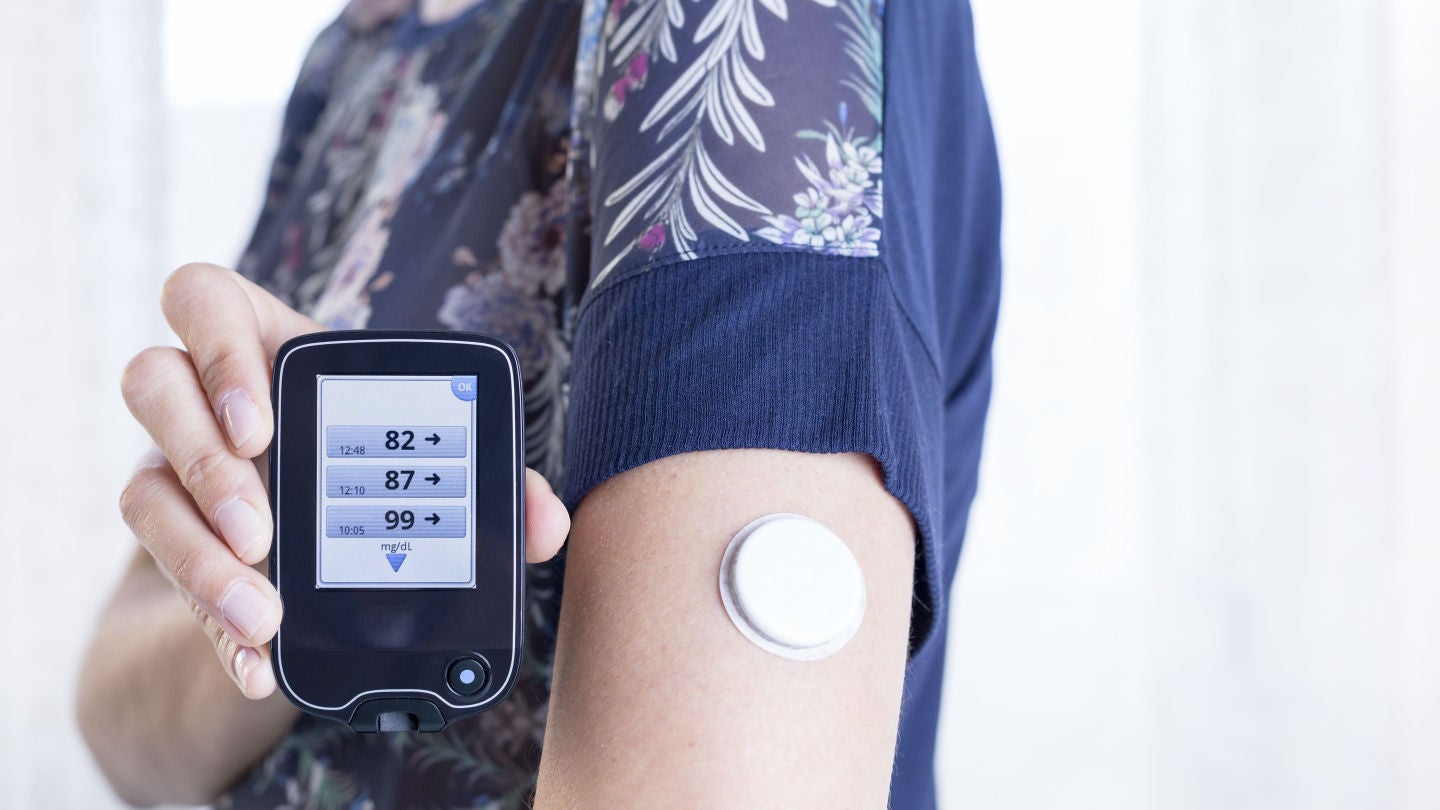
Hypoglycemia events in T1D patients will be monitored using a continuous glucose monitor.
The rate of nocturnal hypoglycemic events will be measured as the primary endpoint while adverse events and time spent in hypoglycemia against placebo will be the study’s secondary endpoints.
The first patient of the Phase IIa study is expected to be dosed in the third quarter of this year.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataZucara Therapeutics clinical and regulatory affairs director Susan Peers said: “We are thrilled to have reached this important milestone and eager to initiate our planned Phase II trial for the prevention of nocturnal hypoglycemia, a frequent occurrence for people with insulin-dependent diabetes that is a significant cause of anxiety for both patients and their loved ones.
“Having demonstrated proof-of-concept and ZT-01’s ability to significantly increase glucagon release in patients with T1D, we are looking forward to showing we can prevent potentially dangerous low blood glucose levels overnight.
“We are excited to take the next step in developing what could be the first therapeutic available to prevent night-time hypoglycemia.”





