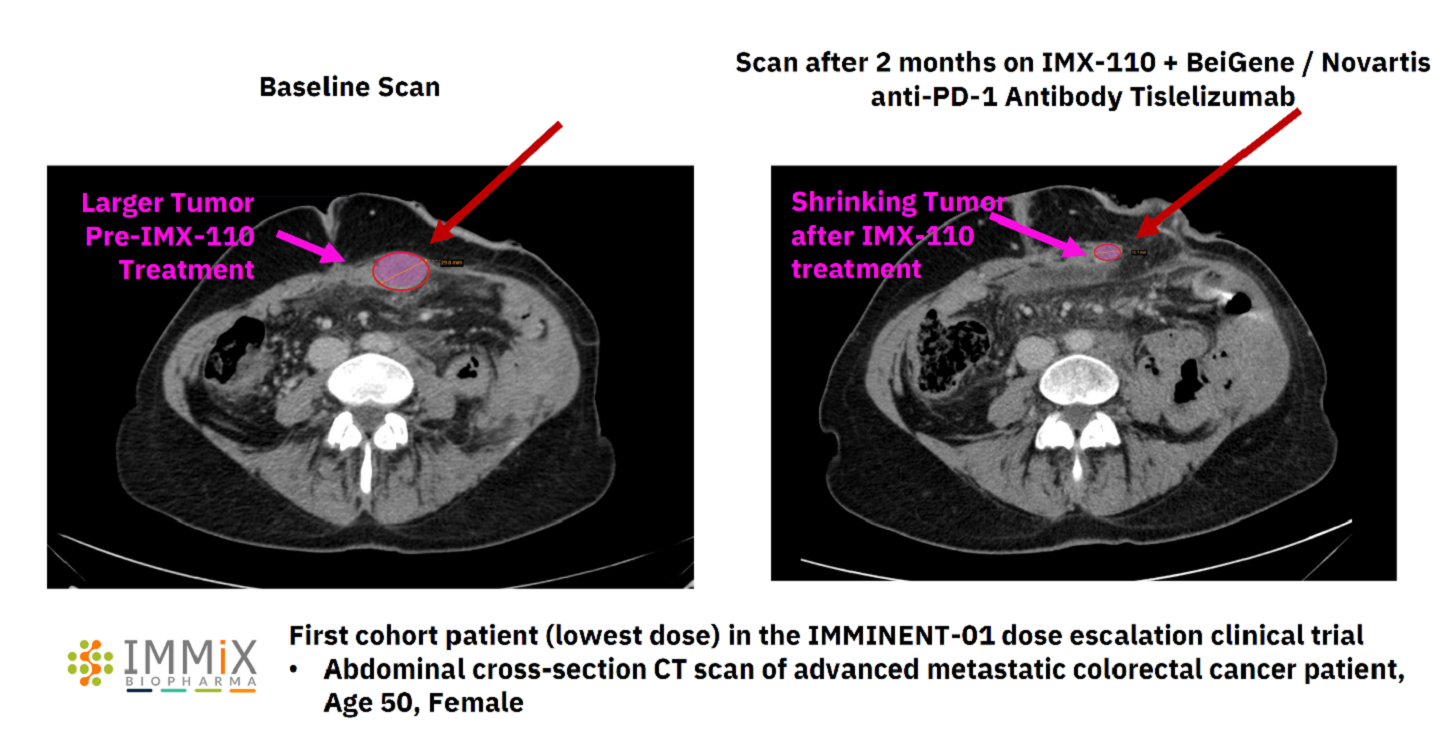
Immix Biopharma has reported early positive interim data from its ongoing Phase Ib/IIa dose escalation clinical trial IMMINENT-01 of IMX-110 for the treatment of advanced metastatic colorectal cancer.
The trial evaluated the combination of tissue specific therapeutic IMX-110 with BeiGene/Novartis’ anti-PD-1 antibody tislelizumab in patients with advanced solid tumours.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Cohorts of three patients will receive escalating IMX-110 doses in the Phase Ib study until the maximum tolerated dose is reached and the recommended dose for Phase II is determined.
Two out of two first evaluable patients who received the lowest dose of IMX-110 demonstrated a 100% tumour shrinkage at two months.
No dose limiting toxicities have been observed in the first treated group enabling Immix to enrol three patients for the next cohort receiving a higher combined dose .
Phase IIa study expects to enrol 30 patients with solid tumour indications.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataEvaluation of safety and identifying the maximum tolerated dose and recommending Phase II dose of IMX-110 plus anti-PD-1 antibody tislelizumab are the primary endpoints of the study.
Secondary endpoints include evaluating the pharmacokinetics and preliminary efficacy of IMX-110 plus anti-PD-1 antibody tislelizumab.
Immix Biopharma CEO Ilya Rachman said: “These initial results potentially validate the scientific rationale for the promise of IMX-110 to unlock our immune system’s ability to fight cancer.”





