Sareum Holdings has received approval for its application submitted for initiation of a Phase I clinical trial of SDC-1801 in Australia.
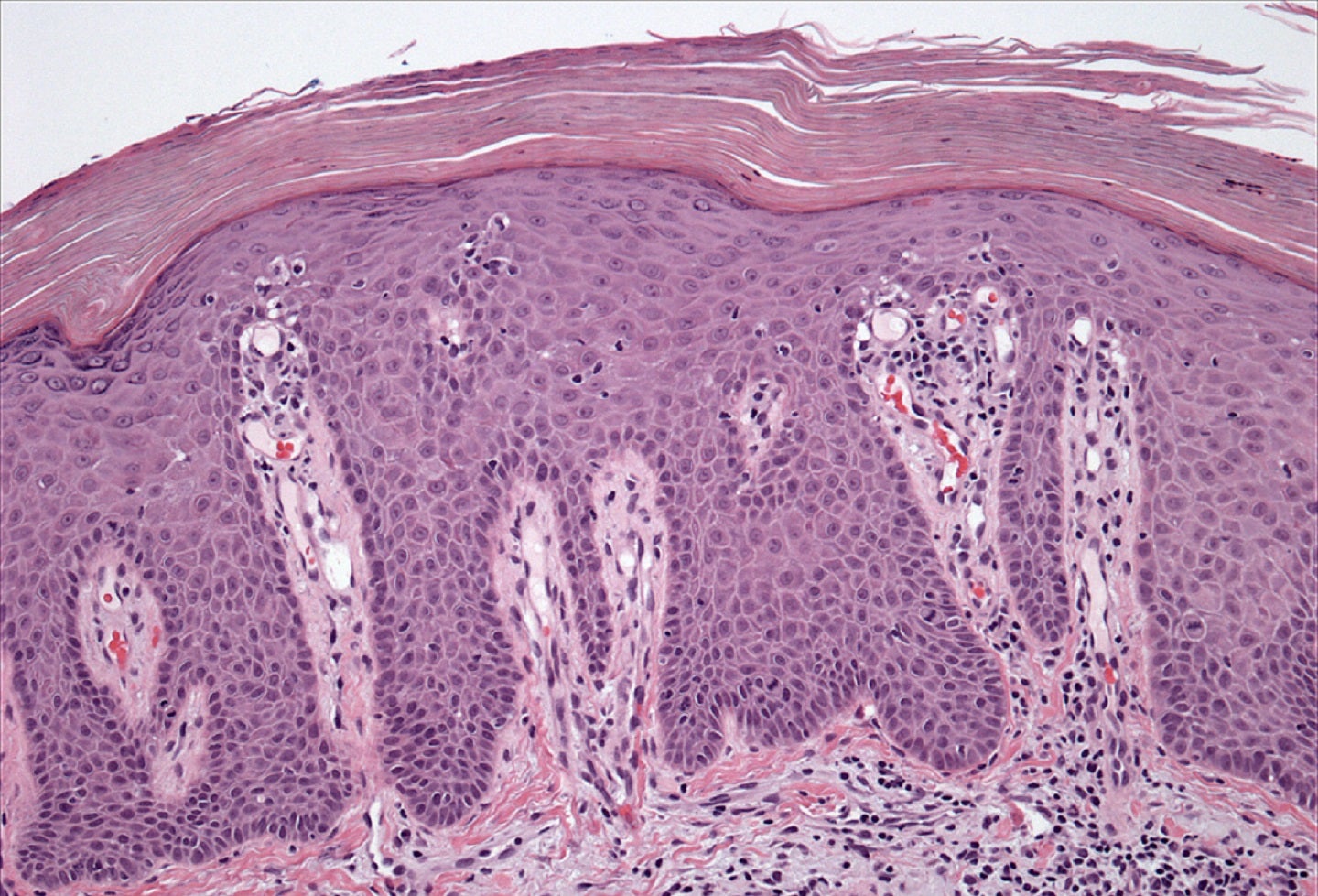
Being developed as a potential new treatment for various autoimmune diseases, SDC-1801 mainly focuses on treating psoriasis, an autoimmune condition that affects the skin.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The study will examine the pharmacokinetic properties along with safety of an oral formulation of SDC-1801 in ascending doses given to healthy participants.
Upon receiving satisfactory safety data from the study, the company plans to initiate a Phase Ib trial next year in patients with psoriasis.
A TYK2/JAK1 inhibitor, SDC-1801 has strong clinical validation in treating psoriasis and psoriatic arthritis and showed benefits in maintaining a healthy immune system.
Sareum CEO Dr Tim Mitchell said: “The approval of this application is a very important step for Sareum and we are now ready to bring our lead asset into clinical development.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We are very excited about the potential of SDC-1801, which we believe could offer superior efficacy compared to other currently-available small molecules for psoriasis and which has demonstrated a good safety profile in preclinical studies.
“We look forward to initiating our Phase I trial in the coming weeks.”
The application has been acknowledged by Australia’s medicines regulator, the Therapeutic Goods Administration, and approved by the Human Research Ethics Committee.





