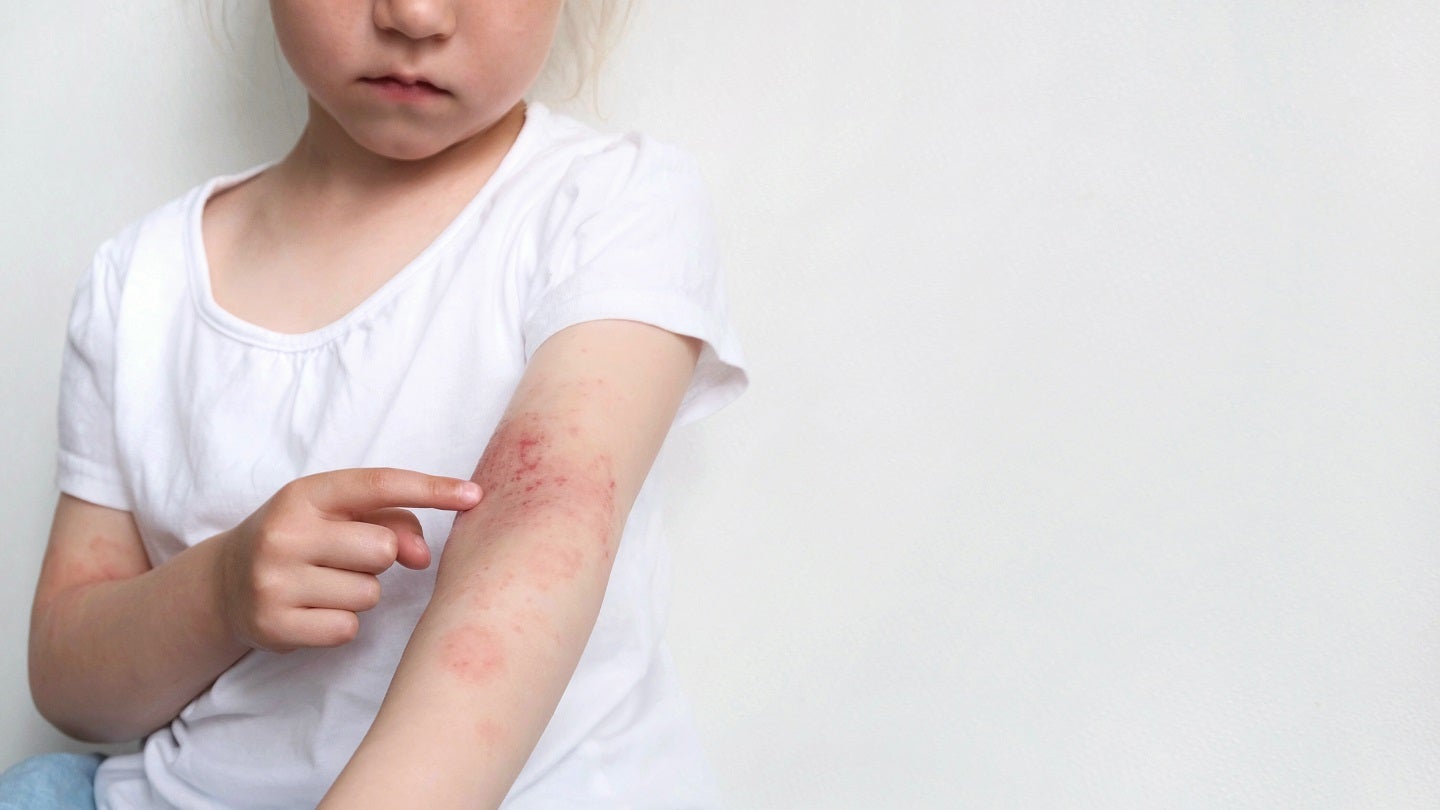
Incyte has reported that its Phase III TRuE-AD3 study of ruxolitinib cream (Opzelura) for the treatment of atopic dermatitis (AD) in children has met its primary endpoint.
The primary endpoint was determined using the Investigator’s Global Assessment Treatment Success (IGA-TS), defined as an IGA score of zero (clear) or one (almost clear), with at least a two-point improvement from baseline at week eight.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The pivotal, randomised, vehicle-controlled study assessed the safety and efficacy of the JAK1/JAK2 inhibitor ruxolitinib against a vehicle (non-medicated cream) in children with AD.
It enrolled over 300 patients aged above two to below 12 years who were diagnosed with AD for at least three months and were suitable for topical therapy.
They were randomised into a 2:2:1 ratio to receive twice daily (BID) formulations of ruxolitinib cream 0.75%, 1.5%, or a vehicle.
Participants in the vehicle group were blindly re-randomised in a 1:1 ratio into either of the active treatment groups.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAt week eight, patients who completed an efficacy assessment were eligible to participate in the 44-week long-term safety treatment extension period.
Secondary endpoints of the trial include the proportion of patients achieving an improvement of at least 75% from baseline measured by the Eczema Area and Severity Index (EASI75) score.
They also include the proportion of participants with at least a four-point improvement in the itch numerical rating scale (NRS).
In addition, the duration, frequency, and severity of adverse events related to ruxolitinib cream will be assessed in the AD study.
Incyte inflammation and autoimmunity group vice-president Jim Lee said: “AD is a chronic, immune-mediated skin condition that affects about 13% of all children in the US.
“We have already seen the benefit that ruxolitinib cream can have among adult and adolescent patients with atopic dermatitis in the TRuE-AD1 and TRuE-AD2 studies, and this new positive data reinforces the potential of ruxolitinib cream to offer children a much-needed effective, non-steroidal topical therapy.
“We look forward to discussing these data with regulatory agencies to determine next steps.”





