Aro Biotherapeutics has enrolled the first subject in its Phase I clinical trial of ABX1100 to treat Pompe disease.
Launched in Canada, the double-blinded, placebo-controlled, single ascending dose part will analyse ABX1100 in healthy subjects.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It will evaluate the tolerability, safety, pharmacokinetics and several pharmacodynamic biomarkers to demonstrate target engagement.
The company plans to report trial findings next year.
Aro Biotherapeutics president and CEO Susan Dillon said: “Our Phase I study of ABX1100 provides an excellent opportunity to demonstrate the potential of our Centyrin-siRNA platform, for targeting siRNAs to tissues beyond the liver.
“We are proud of the work our team has accomplished from discovery through initial clinical testing of ABX1100 and are motivated by our shared goal of bringing a new therapy with unsurpassed efficacy, safety and durability to patients with Pompe disease.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataABX1100 comprises a CD71 receptor attaching Centyrin coupled with a small interfering ribonucleic acid (siRNA).
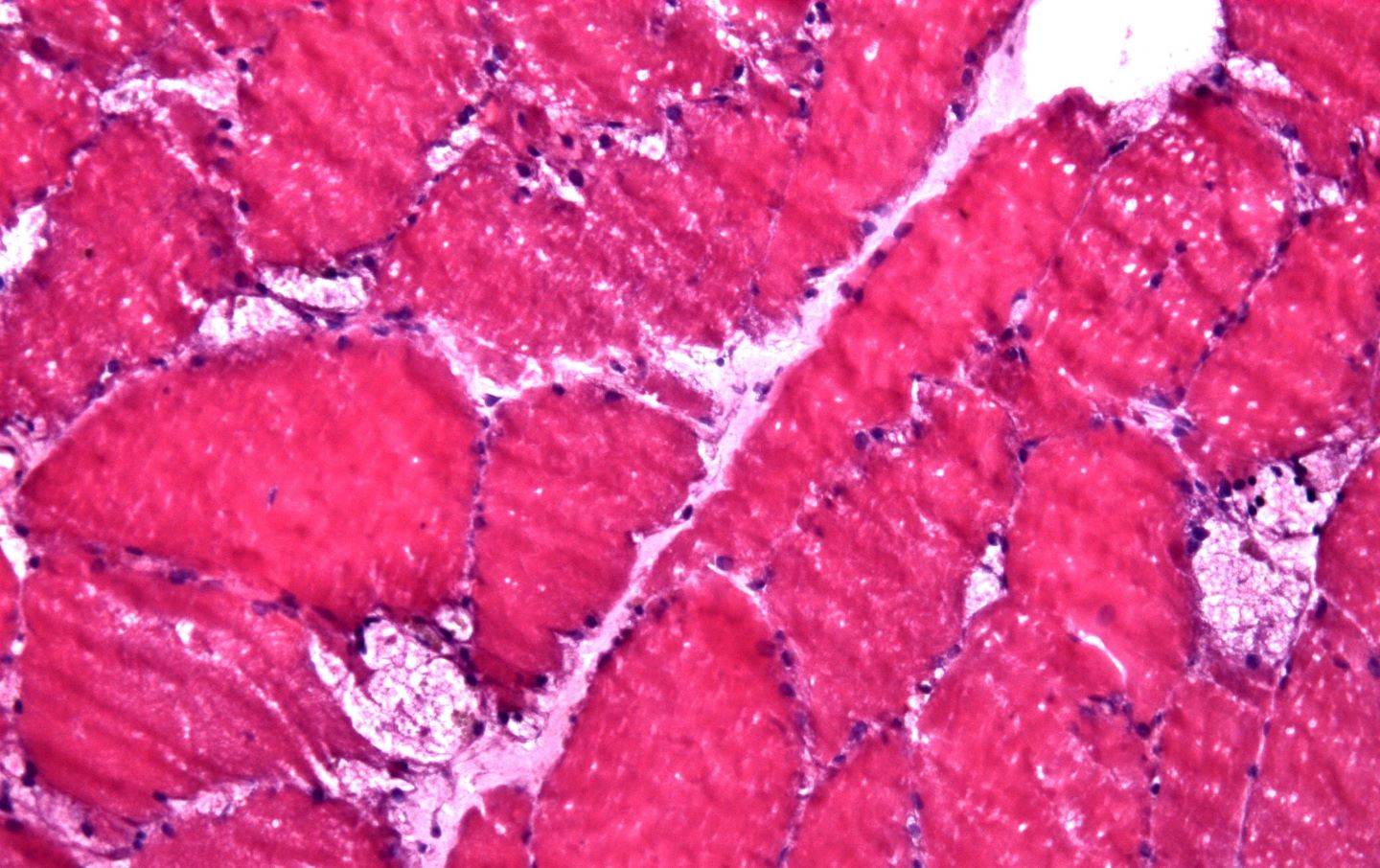
According to preclinical findings from the Pompe mouse model, ABX1100 was demonstrated to cut down levels of additional-hepatic glycogen by delivering a siRNA targeting Glycogen Synthase 1 (Gys1) to the muscle.
Gys1 is the main enzyme that regulates the glycogen synthesis in muscle.
The US Food and Drug Administration (FDA) earlier granted orphan drug and rare pediatric disease designation for the therapy.
A rare, inherited lysosomal storage disease, Pompe disease causes toxic glycogen accumulation.





