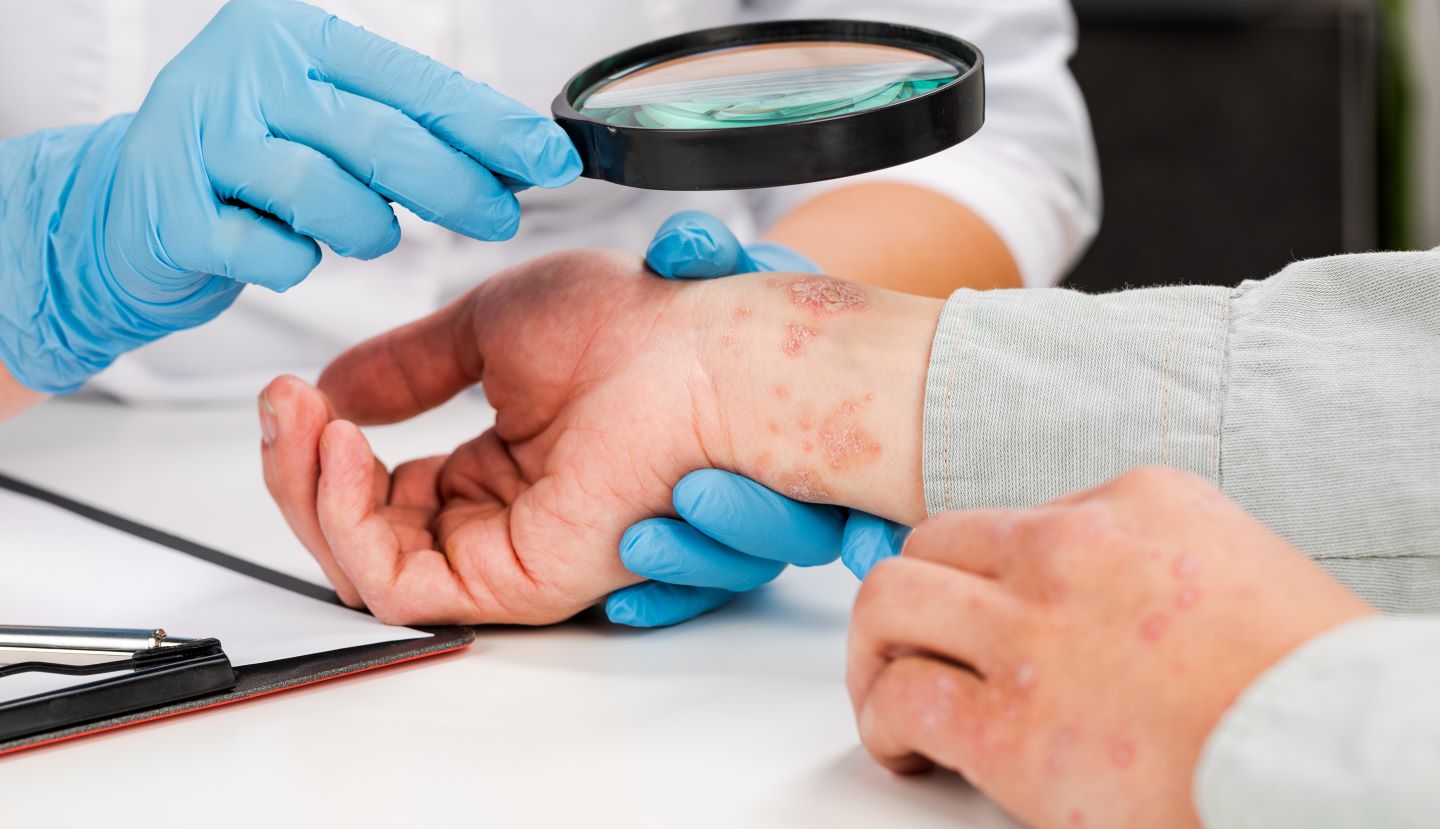
Arctic Bioscience has announced the completion of subject recruitment in the HeROPA Phase IIb clinical trial of new oral drug candidate, HRO350, to treat mild-to-moderate psoriasis.
In January last year, the company received approval in the UK for the Clinical Trial Application (CTA) to launch this Phase IIb trial of HRO350.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
In March, the company reported that the CTA was approved by regulatory agencies in Norway, Finland, Germany, and Poland.
This trial has successfully enrolled the planned 519 patients and is currently being conducted across five European countries, including the UK, Germany, Poland, Finland, and Norway.
The double-blind, randomised, placebo-controlled HeROPA study is designed to evaluate the safety and efficacy of HRO350. The recruitment process has been competitive, with more than 60 sites actively enrolling patients.
In the trial, final participants will be given their respective treatment doses in the coming weeks.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe preliminary trial data readout is expected in mid-2024, after all subjects complete a six-month treatment period.
Arctic Bioscience focuses on the development and marketing of pharmaceutical and nutraceutical products using bioactive marine compounds.
HRO350 is under development for psoriasis patients to address the requirements of a large patient population seeking new therapies with a beneficial safety profile.
In addition to pharmaceuticals, Arctic Bioscience also markets nutraceuticals worldwide.
These products are available as bulk ingredients and finished goods under the ROMEGA brand.
Arctic Bioscience CEO Christer Valderhaug said: “We are very pleased to announce that our HeROPA clinical trial is now fully recruited.
“This is a major milestone for Arctic Bioscience and a result of great team effort by the employees in Arctic Bioscience, our CRO Smerud Medical Research International and the 60 plus clinics participating in the trial.
“With this, we have taken another huge step forward in our medical program within mild-to-moderate psoriasis. After all patients have been dosed for six months, the primary endpoint will be read out.”





