Investigators at Mass General Brigham in Massachusetts, US, have reported outcomes from a study of a new immunotherapy for skin cancer prevention.
The therapy combines topical calcipotriol with 5-fluorouracil (5-FU) and has been shown to eliminate precancerous lesions and prevent squamous cell carcinoma (SCC) for up to five years post-treatment.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It activates specific components of the adaptive immune system, particularly clusters of differentiation 4 +(CD4+) T helper cells.
The open-label clinical trial was led by Shawn Demehri from Massachusetts General Hospital’s Department of Dermatology and the Krantz Family Center for Cancer Research, alongside his team.
It enrolled 18 subjects with qualifying pre-cancerous skin lesions.
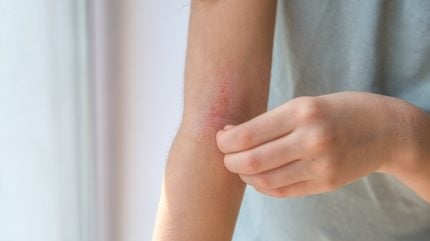
Subjects applied a therapy that combines 0.0025% calcipotriol and 2.5% 5-FU to affected areas twice a day for six days.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPost-treatment evaluations showed that the immunotherapy eliminated 95% of pre-cancerous spots on subjects’ faces, with all facial lesions cleared in seven out of ten subjects.
In addition, 82% of spots on the scalp and more than 65% on both upper extremities were cleared.
Adverse events were limited to inflammation and redness, which resolved within four weeks, while healthy skin remained unaffected.
Demehri said: “One of the unique challenges with squamous cell carcinoma is that individuals who develop it are at an increased risk of developing multiple new lesions over time. This makes prevention an essential part of care.
“We found that this drug combination prevents cancer through a mechanism distinct from those used by current immunotherapies, suggesting that these drugs may treat and prevent cancer via distinct mechanisms.”
Demehri is currently working on a multi-centre trial to determine if immunocompromised patients, such as organ transplant recipients who are at a greater risk for skin cancer, will benefit similarly from this treatment.
His team is also exploring how this trial’s identified mechanism could be applied to other immunotherapies to prevent additional forms of malignancies.





