OSE Immunotherapeutics, a biotechnology company that develops immunotherapies for cancer and autoimmune diseases, and One2Treat, a fast-growing technology company transforming how the patient voice is integrated into clinical development, have won the 2025 Clinical Trials Arena Excellence Award for Innovation in Endpoint Design for a practical idea: involve investigators upfront to define what matters most, then use those priorities to shape a multidimensional endpoint.
In ARTEMIA—a global Phase III trial comparing OSE2101 (Tedopi®), a neoepitope-based cancer vaccine, with docetaxel in HLA-A2 positive metastatic non-small cell lung cancer. OSE Immunotherapeutics partnered with One2Treat and used the One2Treat Voice platform to collect preferences quickly and consistently. In March 2025, 29 investigators completed a short, secure exercise judging simulated patient cases and identifying which outcomes should carry the most weight. Those inputs were translated into a pre-specified exploratory analysis that combines overall survival, quality of life, disease control, and safety into a single, interpretable measure of the Net Treatment Benefit.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Bringing real-world clinical judgment into endpoint selection
The innovation lies in making clinical judgment part of clinical trial design, rather than an afterthought. Investigators viewed anonymized, simulated patient pairs and chose who was better overall, noting which outcome drove each choice. This simple format captured the real trade-offs clinicians make every day and turned it into structured data tied to the protocol’s outcomes.
Speed and ease strengthened the result. In one week in March 2025, 29 investigators completed the exercise in about nine minutes each via a secure link.
The group settled on a clear priority order: overall survival (OS), EORTC QLQ-C30 global quality of life (QoL), progression-free survival (PFS), side-effect burden, and serious adverse events (SAEs). OS was selected as the top outcome in 72% of choices. The platform also captured practical thresholds that make the differences meaningful: three months for OS, one level for QoL, and six months for PFS. Physical and role functioning were considered but did not make the final five, keeping the focus on the outcomes investigators judged most relevant for decisions.
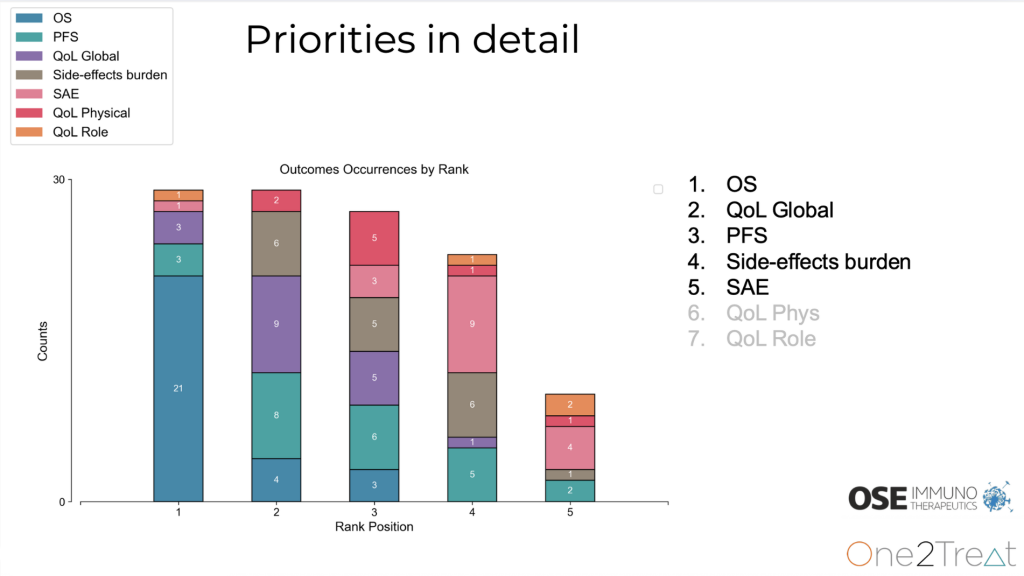
By moving from informal feedback to a transparent, reproducible process, the collaboration gave endpoint definition a clearer clinical foundation.
A multidimensional, interpretable measure of treatment impact
ARTEMIA keeps overall survival as the primary endpoint. The added innovation is a pre-specified exploratory analysis—Net Treatment Benefit (NTB)—that combines multiple outcomes into one readout. Using Generalized Pairwise Comparisons, each patient on Tedopi® is compared with each patient on docetaxel, following the investigator-defined order and thresholds. If one patient lives at least three months longer, that counts toward survival; if not, the comparison moves to global QoL, then to PFS, and, if needed, to side-effect burden and SAEs.
This method produces a single, straightforward probability: the chance that a randomly selected patient on Tedopi® has a better overall outcome than a randomly selected patient on docetaxel, based on the agreed priorities. It makes trade-offs visible and avoids piecemeal interpretation across separate endpoints. For decision-makers, it offers a pre-specified, transparent way to understand overall impact. The clarity and rigor of this multi-outcome assessment were key factors behind the award.
Speed, scale, and a replicable framework with minimal operational burden
The One2Treat Voice approach worked because it was rapid, lightweight, and scalable. Investigators completed the One2Treat Voice exercise in minutes, and results were compiled in days, leaving trial operations uninterrupted. The adaptive questionnaire and simulated cases reduced burden while still capturing nuanced clinical preferences.

The process is also repeatable. It is digital, adaptive, and outcome-agnostic, so it can be applied in other studies where considering the overall treatment effect across several outcomes is important. The steps are clear: elicit preferences, set meaningful thresholds, and link them directly to a predefined NTB analysis. That end-to-end pathway turns assumptions into explicit design inputs and provides a template others can follow. The ability to scale this method without adding complexity was another important reason for the recognition.

“Collaborating with OSE Immunotherapeutics on defining a prioritized list of outcomes for this innovative endpoint has been a meaningful step forward. By grounding multi-dimensional endpoint design in the perspectives of investigators, we’re helping ensure that trial design reflects what really matters for both patients and clinical decision-makers.”
– Sebastien Coppe, CEO, One2Treat
About OSE Immunotherapeutics
OSE Immunotherapeutics is an integrated biotechnology company focused on developing and partnering therapies to control the immune system for immuno-oncology and immuno-inflammation.
About One2Treat
One2Treat is dedicated to transforming the way biopharmaceutical companies design, analyze and interpret randomized clinical trials, and how the overall medical value of a treatment may be communicated. Through its innovative software platform and patient-centered methodologies, One2Treat helps sponsors incorporate the patient’s voice into early trial design, endpoint selection, and treatment value assessment. By aligning clinical evidence generation with patients and clinicians’ needs, One2Treat supports more meaningful clinical trials, efficient decision making and enhanced regulatory discussions, leading to faster market access.
Contact Details
Sebastien Coppe, CEO
Links:



