PALSONIFY™ (paltusotine) is a first-in-class, nonpeptide somatostatin receptor type 2 (SSTR2) agonist indicated as a first-line treatment for adults with acromegaly who either did not achieve satisfactory results from surgery or for whom surgery is unsuitable.
Developed by Crinetics Pharmaceuticals, PALSONIFY offers a convenient, once-daily oral alternative to injectable therapies.
PALSONIFY is available as biconvex oval tablets in 20mg (pink) and 30mg (yellow) strengths.
Regulatory approvals for PALSONIFY
Paltusotine received orphan drug designation from the US Food and Drug Administration (FDA) for the treatment of acromegaly in July 2020.
In September 2024, Crinetics submitted a new drug application (NDA) to the FDA for paltusotine as a treatment and long-term maintenance therapy for acromegaly, which was subsequently accepted in December 2024.
PALSONIFY was approved by the FDA in September 2025, with commercial availability commencing in October 2025.
The European Medicines Agency (EMA) granted orphan drug designation for paltusotine in February 2025 and validated the marketing authorisation application (MAA) in March 2025.
The MAA is currently under review by the Committee for Medicinal Products for Human Use (CHMP), with an opinion expected in the first half of 2026.
Additionally, paltusotine is being developed in Japan through a partnership between Crinetics and Japanese pharmaceutical company Sanwa Kagaku Kenkyusho.
Acromegaly causes and symptoms
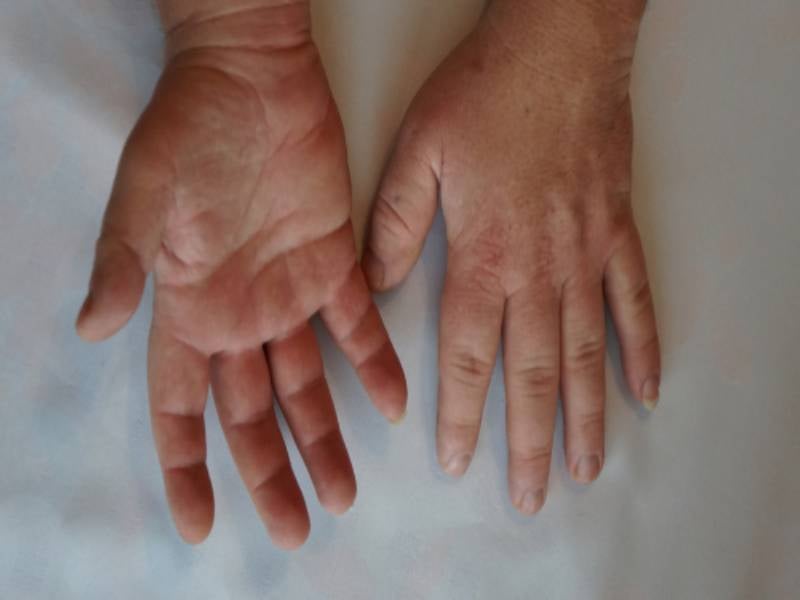
Acromegaly is a rare disease caused by a benign pituitary adenoma that leads to excess secretion of growth hormone (GH), triggering elevated insulin-like growth factor 1 (IGF-1) levels.
Symptoms include joint pain, fatigue, abnormal growth of hands/feet, facial changes, and organ enlargement.
Surgical removal of pituitary adenomas is generally the first-line treatment for most patients with acromegaly.
For those who are not suitable candidates for surgery or require additional therapy, somatostatin receptor ligands (SRLs) are the main pharmacological option. These are typically administered as intramuscular or deep subcutaneous depot injections.
However, many patients do not achieve full disease control, and the monthly injections, often given with large-gauge needles, can cause significant pain and local injection site reactions.
PALSONIFY’s mechanism of action
Paltusotine mimics the effects of the endogenous hormone somatostatin by reducing the secretion of GH and IGF-1. Its action is mediated through highly selective agonism of SSTR2, more than 4,000-fold greater selectivity versus other somatostatin receptor subtypes, with minimal or no binding to the other SST receptors.
In functional assays, paltusotine activated human SSTR2 and suppressed cyclic adenosine monophosphate accumulation, showing an average half-maximal response (EC50) of 0.25 nanomolar (nM).
Clinical trials on PALSONIFY
PALSONIFY’s approval was supported by the PATHFNDR-1 and PATHFNDR-2 Phase III trials, which evaluated the safety and efficacy of the drug in adults with acromegaly, both those who have received prior treatment and those who are treatment‑naive.
The primary endpoint of the studies was the proportion of patients achieving IGF-1 ≤1.0× upper limit of normal (ULN) compared to placebo.
PATHFNDR-1 was a randomised, double-blind, placebo-controlled study, which enrolled 58 acromegaly patients switching from injectable SRLs, randomising them to paltusotine or placebo for 36 weeks, followed by an optional open‑label extension.
At week 36, 83% of paltusotine achieved an IGF-1 level ≤ 1.0 times the ULN compared to 4% for those administered with placebo.
PATHFNDR-2 was a randomised, double-blind, placebo-controlled study, with a 24-week treatment phase, which enrolled 111 participants with acromegaly who had not received pharmacological treatment. The study included an optional open-label extension.
At week 24, 56% of paltusotine-treated patients achieved an IGF-1 level ≤ 1.0 times the ULN compared to 5% of those administered with placebo.
Participants additionally experienced marked decreases in acromegaly-related signs and symptoms, as assessed by the Acromegaly Symptom Diary (ASD).
Common adverse events reported during the trials included diarrhoea, abdominal pain, nausea, decreased appetite, sinus bradycardia, hyperglycaemia, palpitations, and gastroenteritis.
Clinical trial for carcinoid syndrome
Paltusotine is also under investigation for the treatment of carcinoid syndrome, which affects some patients with neuroendocrine tumours (NETs).
NETs are rare, slow-growing cancers that typically originate in the digestive tract. Carcinoid syndrome develops when these tumours metastasise to the liver, leading to symptoms such as diarrhoea and flushing.
A Phase II randomised, open-label, parallel-group, multi-centre study for this indication was conducted involving 36 participants with carcinoid syndrome.
The trial demonstrated that reductions in the frequency and severity of symptoms were observed within two weeks of starting paltusotine, with these improvements maintained over an eight-week period in both untreated patients and those switching from SRL therapy.
The results also showed a rapid and sustained decrease in flushing episodes and bowel movement frequency, which are the most common symptoms of carcinoid syndrome.
Following these positive findings, a global Phase III multi-centre, randomised, double-blind, placebo-controlled clinical trial named CAREFNDR has been initiated to further assess paltusotine for the treatment of carcinoid syndrome.
The trial will recruit 141 patients aged 18 or above with carcinoid syndrome.