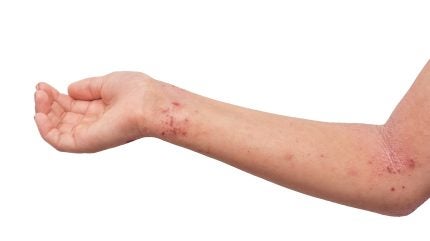
Alphyn Biologics has completed $25m in a Series B funding round to support the global Phase IIb trial of its lead candidate, Zabalafin Hydrogel, for atopic dermatitis (AD).
The proceeds will also support the initiation of a second Phase II clinical programme for molluscum contagiosum virus (MCV) and expand the drug raw material supply.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
QCA Investment Group spearheaded the twice-oversubscribed round, which saw participation from existing and new investors.
Zabalafin Hydrogel is under development as a treatment targeting the interconnected drivers of AD, including itch, inflammation, dry skin, and bacteria.
The drug is also being investigated for its potential to address the virus, inflammation, itch, dermatitis, and bacterial infection associated with MCV.
According to Alphyn, Zabalafin Hydrogel is expected to offer sustained management along with long-term control of these conditions.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe company noted that, for MCV, the hydrogel could become the first direct antiviral drug that is both effective and safe, offering a new treatment option for paediatric patients affected by the condition.
For AD, Zabalafin Hydrogel is designed to directly treat the immuno-inflammatory component, pruritus, bacterial involvement, and xerosis.
Alphyn Biologics CEO Neal Koller said: “We are incredibly grateful for the support of our current and new investors. This financing reflects their confidence in our multi-target therapeutic drug platform and our unique and highly differentiated drug candidates for atopic dermatitis and the molluscum contagiosum virus.
“We are well-positioned to rapidly advance Zabalafin Hydrogel towards our next key milestones – two pivotal Phase III trials – while broadening our pipeline of breakthrough therapies for skin diseases.”
The US-based company, which became operational in 2020, has subsidiaries in Austria and Australia, and has so far secured $34m.





