Jascayd® (nerandomilast/BI 1015550) is a phosphodiesterase 4 (PDE4) inhibitor developed by Boehringer Ingelheim for the treatment of adults with idiopathic pulmonary fibrosis (IPF) and progressive pulmonary fibrosis (PPF).
The drug is described as the first and only preferential phosphodiesterase 4B (PDE4B) inhibitor approved for the treatment of IPF in adults.
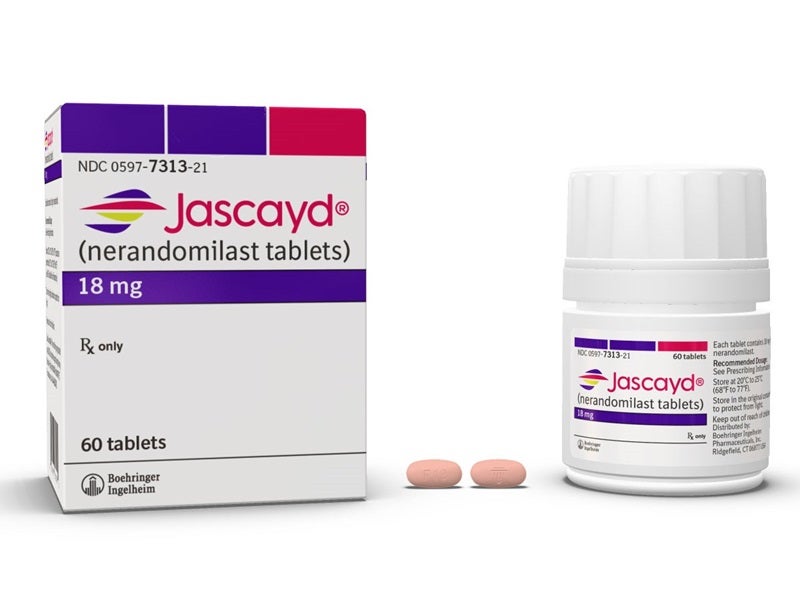
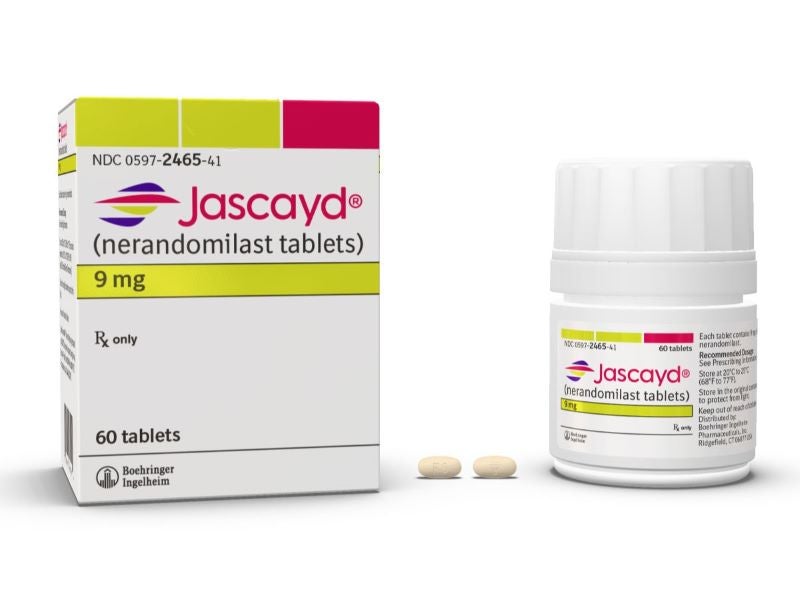
Jascayd is available as nine milligram (mg), light yellow-coloured and 18mg, light red-coloured, oval, biconvex, film-coated tablets.
Regulatory approvals
Jascayd earned Breakthrough Therapy designation from the US Food and Drug Administration (FDA) for IPF in February 2022 and for PPF in April 2025.
The medication received FDA approval as an oral treatment for adults with IPF in October 2025.
In the same month, China’s National Medical Products Administration (NMPA) approved Jascayd for the treatment of adults with IPF.
In December 2025, the FDA and China’s NMPA approved Jascayd for the treatment of adults with PPF.
IPF causes and symptoms
IPF is among the most common forms of progressive fibrosing interstitial lung disease.
The condition is characterised by thickening and stiffening of lung tissue, which can ultimately lead to scarring (fibrosis), which impairs breathing and reduces the transfer of oxygen from the lungs to the rest of the body.
Common symptoms include shortness of breath during activity, a persistent dry cough, chest discomfort, fatigue and weakness. Although regarded as a rare disease, IPF affects an estimated three million people worldwide.
Jascayd’s mechanism of action
Nerandomilast is a PDE4 inhibitor that, based on in vitro data, shows at least nine-fold greater selectivity for the PDE4B isoenzyme than for PDE4A, PDE4C or PDE4D.
PDE4 hydrolyses and inactivates cyclic adenosine monophosphate (cAMP), a key second messenger involved in regulating fibrotic and inflammatory pathways.
By inhibiting PDE4B, nerandomilast increases intracellular cAMP concentrations and down-regulates the production of pro-fibrotic growth factors and inflammatory cytokines, which are overexpressed in IPF.
This dual anti‑fibrotic and immunomodulatory activity may help to slow the deterioration of lung function in patients with IPF.
Clinical trials on Jascayd
The safety and efficacy of Jascayd were assessed in two Phase III randomised, double-blind, placebo-controlled trials: FIBRONEER-IPF and Trial 2.
In both studies, patients were required to have an IPF diagnosis according to American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/ Latin American Thoracic Association criteria.
The FDA approval of Jascayd was based on the FIBRONEER-IPF trial, which enrolled 1,177 adult patients with IPF, with or without background antifibrotic therapy (nintedanib or pirfenidone).
Patients were randomised 1:1:1 to receive 9mg of Jascayd twice daily, 18mg twice daily, or placebo twice daily, and were treated until the last patient had completed 52 weeks of therapy. The time frames of the blinded treatment phase and the total duration are 91 weeks and 109 weeks, respectively.
The trial’s primary endpoint was the absolute change from baseline in forced vital capacity (FVC) at week 52, expressed in millilitres (ml), comparing Jascayd with placebo.
In the FIBRONEER-IPF clinical trial, patients receiving Jascayd experienced a smaller decline from baseline in FVC than those receiving placebo, and the reduction in decline was statistically significant.
The adjusted mean decline was -106ml with Jascayd 18mg and -122ml with Jascayd 9mg, compared with -170ml in the placebo group. The corresponding treatment differences versus placebo were 64ml and 48ml, respectively.
The most common adverse reactions reported during the trial were back pain, Covid-19, decreased weight, depression, diarrhoea, dizziness, fatigue, headache, nausea, reduced appetite, upper respiratory tract infection and vomiting.
Trial 2 was a 12-week study that enrolled 147 adult patients with IPF, with or without background antifibrotic therapy (nintedanib or pirfenidone). Patients were randomised 2:1 to receive 18mg of Jascayd twice daily or placebo twice daily for 12 weeks.
In the trial, patients who received 18mg of Jascayd twice daily, either alongside background antifibrotic therapy or without it, experienced a 91ml-smaller decline in FVC at week 12 than those given a placebo.