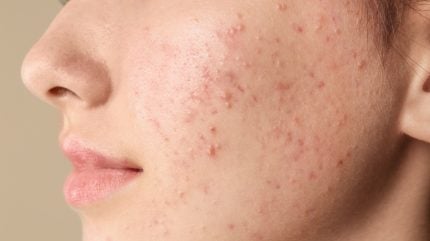
Ascletis Pharma has announced positive top line results from its Phase III open-label trial assessing the long-term safety of denifanstat (ASC40) in patients with moderate to severe acne vulgaris.
The multi-centre study took place in China and involved 240 patients, all of whom previously received either denifanstat or placebo for 12 weeks before receiving denifanstat once daily for 40 weeks.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Its primary endpoints included the incidence of treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), and discontinuations due to adverse events (AEs).
Denifanstat showed tolerability and a favourable safety profile, and most TEAEs were mild (grade one) or moderate (grade two). The company reported no denifanstat-related grade three or four AEs, no related SAEs, and no deaths during the study period.
In June 2025, denifanstat achieved all primary, key secondary, and secondary endpoints in its previous double-blind, placebo-controlled, randomised Phase III trial involving 480 patients, also for the treatment of moderate to severe acne vulgaris.
Denifanstat operates through dual mechanisms for acne treatment: inhibition of de novo lipogenesis (DNL) in human sebocytes to directly reduce sebum production and inhibition of inflammatory responses by decreasing cytokine secretion and Th17 differentiation.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAscletis holds exclusive rights to denifanstat, a once-daily oral fatty acid synthase (FASN) inhibitor, in Greater China through a licensing agreement with Sagimet Biosciences.
Recently, the China National Medical Products Administration accepted the New Drug Application for denifanstat for acne.
In June 2025, Sagimet Biosciences’ denifanstat succeeded in a Phase III trial. The Chinese study conducted by Ascletis saw denifanstat achieve a 33.2% treatment success in patients with acne vulgaris taking the oral medication for 12 weeks.





