MediciNova has started patient enrolment in a Phase I/II clinical trial evaluating the combination of MN-166 (ibudilast) and temozolomide (TMZ, temodar) for the treatment of recurrent glioblastoma (GBM).
The trial aims to investigate the safety and tolerability of MN-166 (ibudilast) in combination with TMZ.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Part I of the trial is a dose-escalation phase, while Part II features a fixed-dose phase. The two parts are expected to enrol 15-18 and 32 subjects respectively.
MediciNova intends to assess the safety and tolerability of MN-166 (ibudilast) in combination with TMZ in Part I and establish the MN-166 (ibudilast) dose.
Part II is designed to study the efficacy of MN-166 (ibudilast) and TMZ combination in patients with recurrent GBM as assessed by the proportion of patients who are progression-free at six months.
Additional outcome measures of the trial are the evaluation of overall survival, response rate, and median six-month progression-free survival.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataHarvard Medical School Neurology professor Patrick Wen, and University of New South Wales, Australia Lowy Cancer Research Centre Biomarkers and Translational Research head and associate professor Kerrie McDonald are the trial’s principal investigators.
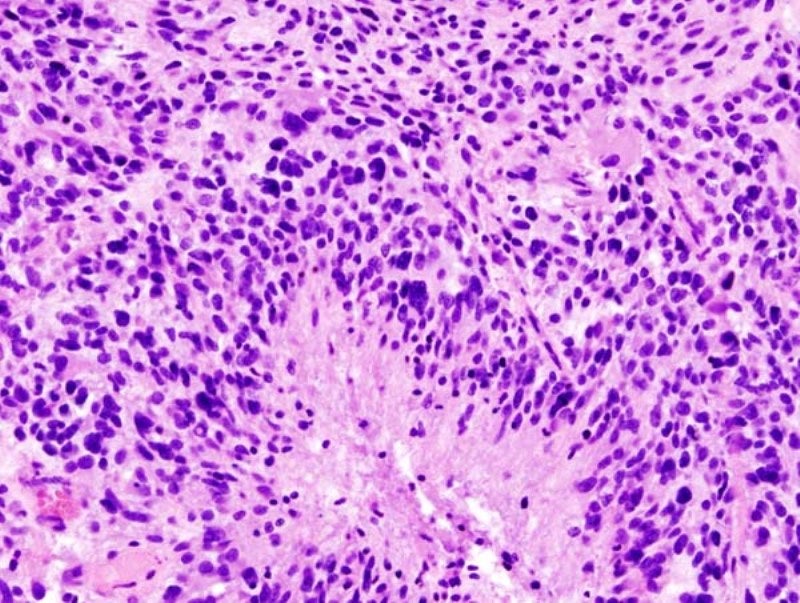
McDonald said: “Earlier studies indicate that macrophage migration inhibitory factor (MIF) and phosphodiesterase (PDE)-4 may factor in proliferation of GBM tumours.
“MIF was found to be highly expressed within GBM cells, and especially around necrotic areas and in close proximity to blood vessels.
“Ibudilast in combination with TMZ resulted in significant blockage of MIF expression, increased apoptosis, and longer survival in vivo.”
MediciNova is currently developing MN-166 for the treatment of progressive multiple sclerosis (MS) and other neurological diseases including amyotrophic lateral sclerosis (ALS), substance abuse / addiction, chemotherapy-induced neuropathy, and GBM.





