Cabozantinib is an anticancer drug for inhibiting tumour growth, angiogenesis and metastasis. It is orally bioavailable and has a potential anti-neoplastic activity. The small anti-cancer compound targets multiple receptor tyrosine kinases (RTK) including the vascular endothelial growth factor receptor 2 (VEGFR-2) and hepatocyte growth factor receptor (MET). They are responsible for development and progression of many cancers.
Cabozantinib has been developed by Exelixis, a biotechnology company that develops molecule therapeutics for cancer treatment. The drug received orphan drug designation from the US Food and Drug Administration (FDA) in January 2011.
Exelixis filed a new drug application (NDA) for cabozantinib with the FDA in May 2012.
Medullary thyroid cancer (MTC)
MTC is one of the most common thyroid cancers accounting to about 5% to 8%, according to the American Cancer Society. The parafollicular cells of thyroid cause MTC. About 80% of the cancer is due to sporadic forms and 20% is through inheritance. The activating RET germline DNA mutations occur in patients with inherited form of MTC.
The mutations are also present in DNA of the tumour in about 50% of patients with sporadic MTC. When it is not diagnosed, the cells can metastasise to lymph nodes and other organs. The treatment is also more complex than the other thyroid cancers and has no approved therapies. Common procedures include surgery, chemotherapy and radiation therapy.
Targets
Cabozantinib showed activity against the cancer tumours in pre-clinical studies. The tumours were less aggressive and less invasive upon the treatment, when compared to selective or controlled anti-VEGF treatments.
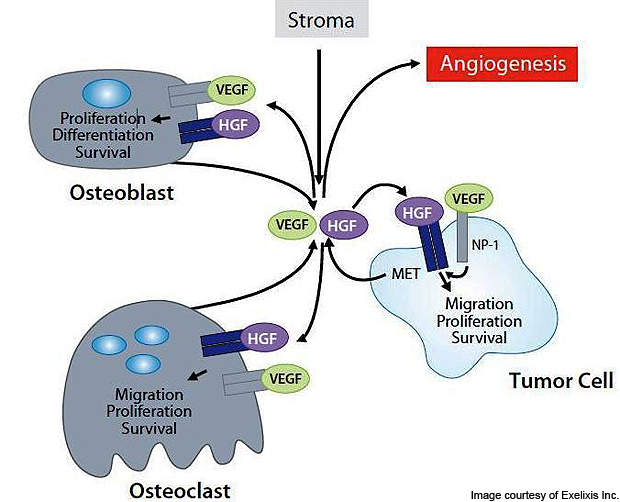
Overexpression of ligand hepatocyte growth factor (HGF) of MET and activation of the MET pathway support the cancer tumours. The VEGFR-2 and MET allow them to overcome hypoxia and stimulate angiogenesis following the inhibition.
Angiogenesis, proliferation, invasion and migration of cells is carried out by the proto-oncogene MET. These biological processes by the receptor tyrosine kinase contribute to the survival, transformation, metastasis and progression of the cancer cells.
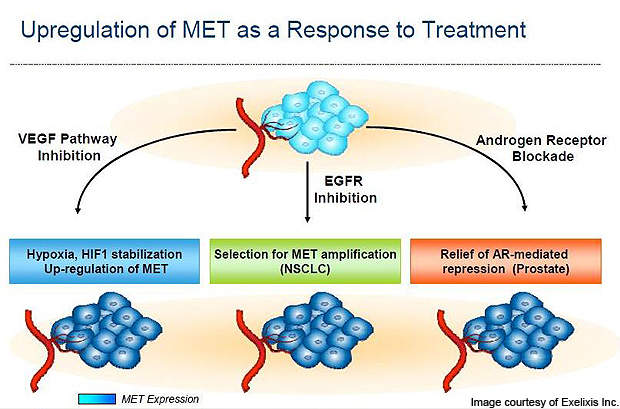
Selective anti-VEGF therapies do not inhibit the MET (c-MET). The invasiveness results in upregulation of MET, which in turn results in evasive or acquired resistance against the VEGF signalling agents. Cabozantinib has the potential to inhibit the MET and improve the outcomes against several indications.
It is also a potential inhibitor of stem cell growth factor, tyrosine-protein kinase receptor (Tie-2) and the FMS-like tyrosine kinase 3.
Cabozantinib clinical trials
Clinical and safety data of the cabozantinib Phase I study were presented at the American Society of Clinical Oncology (ASCO) in 2008. The study was conducted with cabozantinib as a single agent on 69 patients and the results were positive.
A Phase II randomised discontinuation trial was conducted to examine cabozantinib’s activity in a variety of indications, including melanoma, non-small cell lung cancer, small cell lung cancer, hepatocellular carcinoma, pancreatic cancer and metastatic castrate-resistant prostate cancer (mCRPC). The trial enrolled 490 patients, and the interim results of the trial were released in February 2011. Soft tumour regression was seen in all types of cancers with tumour suppression rates of 73% in HCC, 68% in CRPC, 53% in ovarian cancer, 47% in melanoma, 45% in breast cancer, and 40% in NSCLC.
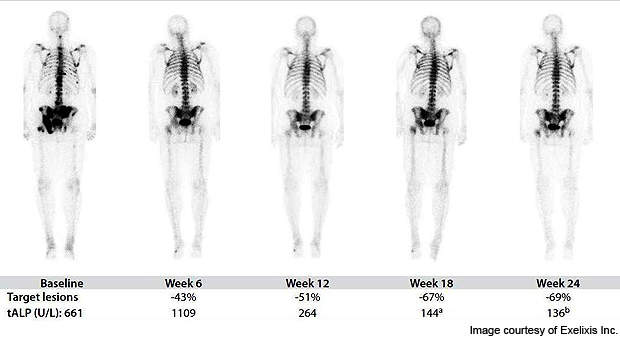
In November 2010, interim data of bone scans on mCRPC patients indicated the drug showed either complete or partial resolution of lesions. The independent review showed the drug to be effective in 19 of 20 patients (95%).
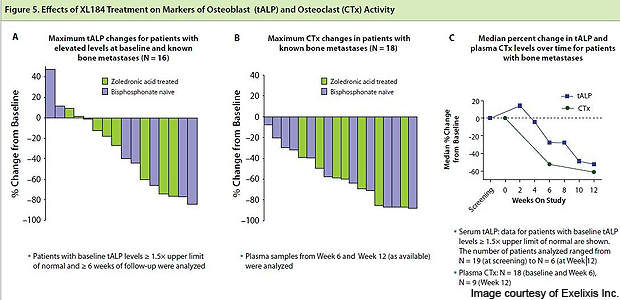
On 17 February 2011, Exelixis announced updated interim data from Phase II clinical trials. Bone scans of 53 patients out of 62 (85%) achieved either complete or partial resolution of metastatic lesions. The drug was also effective in reducing bone pain, improving haemoglobin in patients with anaemia, and tumour regression.
The company is evaluating the Phase I trials on differentiated thyroid cancer and renal cell carcinoma patients. The Phase II study in adults with glioblastoma multiforme and the initial Phase I of glioblastoma with cabozantinib combined with radiation therapy and Temozolomide are also underway. The Phase II trials of the drug in adults with advanced malignancies and Phase Ib/II study in combination with Erlotinib for non-small cell lung cancer are also ongoing.
Primary results from the Phase II glioblastoma multiforme study were announced in May 2009. Results from the Phase II trial in advanced malignancies are expected to be released by December 2012.
Pivotal Phase III clinical trials are being conducted in 200,000 people to treat metastatic, medullary, follicular and anaplastic thyroid carcinoma or locally advanced papillary thyroid cancer.
Exelixis began the pivotal Phase III clinical trial known as EXAM trial on cabozantinib as a potential MTC inhibitor in July 2008. It was a randomised, placebo controlled, double blinded study to test the efficacy of cabozantinib against locally advanced, unresectable, metastatic MTC in 330 patients. The daily oral dose of cabozantinib or placebo was randomised in a 2:1 ratio.
Patients were administered either 25mg or 100mg of cabozanitinib or a gelatin capsule as a placebo. The primary endpoint of the trial was higher rates of progression free survival when compared to the placebo group.
In June 2012, Exelixis announced that the EXAM trial had met its primary end point. The results showed that the patients treated in cabozantinib arm achieved a median PFS of 11.2 months compared four months in placebo arm. The secondary endpoint or the Overall Response Rate (ORR) in the cabozantinib arm was 28% and 0% in the placebo arm.
All the clinical trials are expected to be completed by March 2013.
Marketing commentary
Cabozantinib is the first-in-class potential inhibitor for both MET / VEGFR receptor tyrosine kinases. The orphan drug status will support its development for treatment against various cancers in the US. The status provides marketing exclusivity for seven years, an FDA user fees waive and 50% tax benefits for the qualified clinical trial expenses. Exelixis is also planning to conduct the trials in Europe.