Striverdi Respimat (olodaterol) is an inhalation spray indicated for the maintenance treatment of chronic obstructive pulmonary disease (COPD), including chronic bronchitis and / or emphysema. The drug was discovered and developed by Boehringer Ingelheim Pharmaceuticals.
The US Food and Drug Administration (FDA) approved Striverdi Respimat 5µg spray in August 2014 as a long term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with COPD. The spray is not indicated for treating asthma and acute deteriorations of COPD.
Related content
Anoro Ellipta for Treatment of Chronic Obstructive Pulmonary Disease
Anoro ellipta is a dry powder for inhalation indicated for the treatment of airflow obstructions in patients suffering from Chronic Obstructive Pulmonary Disease or emphysema.
Striverdi Respimat also received marketing authorisation in the European Union as a fast-acting and long-lasting bronchodilator for the maintenance treatment of COPD in October 2013 and Health Canada approved the spray in 2013.
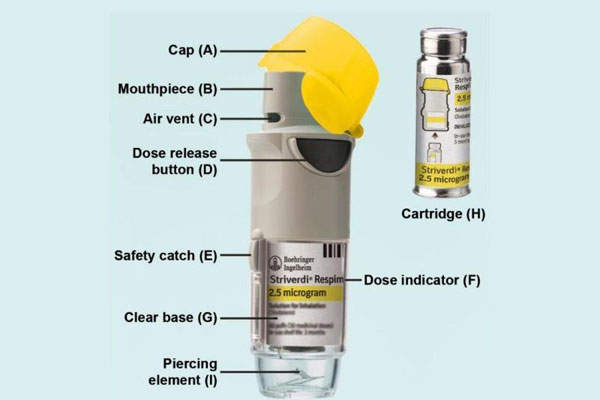
Striverdi Respimat inhalation spray is available in the form of a cartridge filled with a sterile, aqueous solution of olodaterol hydrochloride. The cartridge is a 4.5ml plastic container crimped into an aluminium cylinder and should be used only with the Striverdi Respimat inhaler, which is a pocket-sized handheld oral inhalation device. The device delivers a soft, slow-moving mist of medication from a metred volume of the drug solution through mechanical energy.
Chronic obstructive pulmonary disease (COPD)
COPD is a progressive disease that makes breathing difficult as less air flows in and out of the lungs. It includes chronic bronchitis and emphysema and becomes worse over time.
Cigarette smoking is considered to be a leading cause of COPD, although the disease also affects non-smokers. Other causes of COPD include long term exposure to lung irritants like chemical fumes, dust and air pollution. Symptoms of COPD worsen over time. Some of the most common symptoms include coughing with or without mucus, shortness of breath, wheezing and chest tightness.
COPD is the third leading cause of death in the US. An estimated 26 million Americans, half of them undiagnosed, are affected with COPD.
Striverdi Respimat’s mechanism of action
Striverdi Respimat consists of an active component called olodaterol, a long-acting beta2-adrenergic agonist (LABA), which binds and activates the beta2-adrenoceptors. These activated receptors stimulate the enzymes that are responsible for the synthesis of 3′, 5′-cyclic adenosine monophosphate (cAMP). cAMP, when produced in higher levels, induces bronchodilation by the relaxation of airway smooth muscle cells.
Striverdi Respimat clinical trials
The FDA’s approval for Striverdi Respimat is based on the results of a Phase III clinical trial, which included the data from 48-week and 6-week duration trials.
The study included four pairs of replicate, randomised, double-blind, placebo-controlled trials conducted on 3,533 COPD patients, among whom 1,284 received a 10µg dose and 1,281 receiveda 5µg dose. Two pairs of studies were conducted for 48 weeks and two other pairs of trials were conducted for six weeks. The participants were aged 40 years or older with COPD, a smoking history of at least 10 years and with pulmonary impairment.
Results of the pivotal Phase III clinical studies demonstrated that the 48 week trials in which Striverdi Respimat 5µg was administered once daily in the morning demonstrated significant improvement in lung function compared to the placebo. The drug showed a bronchodilatory treatment effect within five minutes after taking the first dose. It also improved morning and evening PEFR (peak expiratory flow rate) compared to the placebo.
Even in the six week trials, Striverdi showed significant improvement compared to placebo. The 10µg dose showed no additional benefit over the 5µg dose, however.
Mild to moderate adverse reactions including nasopharyngitis, hypertension, dizziness, rash and arthralgia were encountered during the clinical study.
Marketing commentary
Headquartered in Ingelheim, Germany, Boehringer Ingelheim (BI) is one of the world’s leading pharmaceutical companies. BI’s lung health portfolio focuses on addressing the challenges faced by people with lung diseases. The company specialises in developing new treatments for major respiratory ailments such as COPD, asthma, idiopathic pulmonary fibrosis and lung cancer.
Boehringer Ingelheim also produces Spiriva (tiotropium bromide), the first inhaled maintenance treatment for COPD. The product was introduced ten years ago and has become one of the most prescribed maintenance treatments for COPD worldwide.