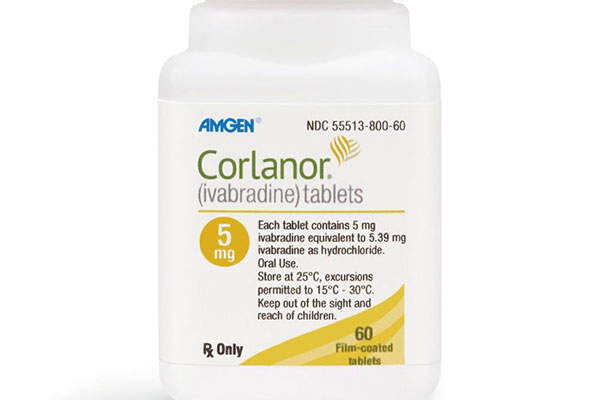
Corlanor (ivabradine) is an oral medication approved to reduce the risk of hospitalisation for worsening heart failure in patients facing chronic heart failure risk due to the lower-left part of the heart not contracting well.
It is the first medicine approved for chronic heart failure in a decade.
The drug was developed by Les Laboratoires Servier. Amgen, in collaboration with Servier, holds the rights to market the drug in the US.
The US Food and Drug Administration (FDA) approved Corlanor (ivabradine) in the US in April 2015. The drug is specifically indicated for patients with stable heart failure, a normal heartbeat with a resting heart rate of at least 70 beats a minute and taking beta blockers at maximum tolerated doses.
The drug was reviewed under the FDA’s priority review programme and was also granted fast track designation.
Prevalence of heart failure in the US
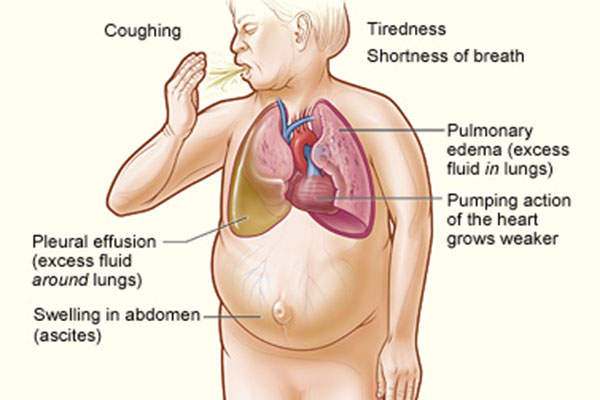
Heart failure is a condition which develops over a period of time due to the heart’s inability to supply enough blood to meet the body’s needs.
The condition worsens as the heart’s pumping action becomes weaker. The most common causes of heart failure include coronary heart disease and high blood pressure.
The condition affects an estimated 5.7 million people in the US, half of who have a malfunctioning left ventricle. The prevalence of heart failure is forecasted to increase by 46% by 2030. Condition-related costs account for $31bn a year and are expected to reach $70bn by 2030.
Corlanor’s mechanism of action
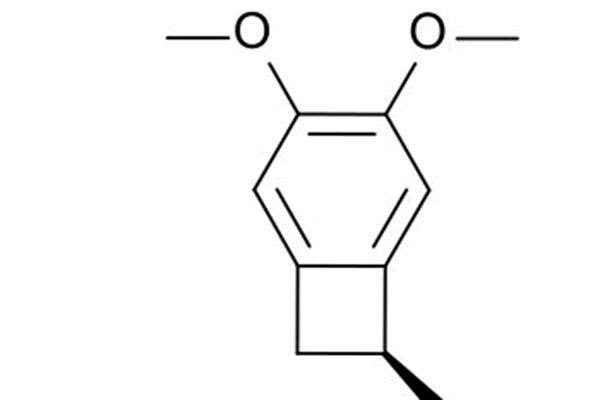
Corlanor contains an active component called ivabradine, which is a hyperpolarisation-activated cyclic nucleotide-gated channel blocker responsible for regulating heart rate.
Ivabradine reduces spontaneous pacemaker activity of the cardiac sinus node, inhibits the If current and slows down heart rate without any effect on ventricular repolarisation.
Corlanor is available as salmon-coloured, film-coated tablets in 5mg and 7.5mg doses for oral administration only.
Clinical trials on Corlanor
CXL-1020 is an investigational therapeutic agent being developed for treating patients with acute decompensated heart failure.
Corlanor’s approval by the FDA was based on results from a Phase III clinical trial known as Shift (systolic heart failure treatment with the If inhibitor ivabradine trial).
Shift was a randomised, double-blind, multi-centre, placebo-controlled study that enrolled 6,505 patients with stable New York Heart Association (NYHA) class II to IV heart failure, left ventricular ejection fraction of 35% and a resting heart rate of at least 70 beats per minute.
The study compared Corlanor with a placebo on top of standard of care (SOC) therapies, including beta blockers in patients with sinus rhythm with reduced left ventricular function and a heart rate greater than 70 beats per minute, with a record of hospitalisation for heart failure within the past 12 months.
Most of the patients were taking SOC therapies including beta blockers, angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARB), diuretics and anti-aldosterone agents.
The study results showed the drug significantly reduced the risk of hospitalisation or cardiovascular death for worsening heart failure with 18% relative risk reduction compared with a placebo.
The most common adverse reactions recorded in the study were bradycardia, hypertension or increased blood pressure, atrial fibrillation, and luminous phenomena or visual brightness.
Marketing commentary
Amgen has more than three decades of experience in developing biotechnology medicines for serious illnesses, The company addresses medical needs of patients with cardiovascular diseases, which are the leading cause of morbidity and mortality worldwide.
The company’s in-house research and development into cardiovascular diseases has helped it build a robust pipeline of investigational medicines for many unmet medical needs, including heart failure and high cholesterol.