Poly adenosine diphosphate ribose polymerase (PARP) inhibitor Rubraca® (rucaparib) is indicated for the treatment of breast cancer susceptibility gene (BRCA) mutation-linked advanced ovarian cancer.
Developed by Clovis Oncology, the drug was approved by the US Food and Drug Administration (FDA) in December 2016 and is available in tablet form for oral administration.
A supplemental new drug application (NDA) was also submitted for Rubraca® as a maintenance therapy for patients with recurrent ovarian cancer in October 2017, which was approved in April 2018.
The marketing authorisation application (MAA) for the drug was submitted to the European Medicines Agency (EMA) in November 2016. The EMA’s Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending the granting of a conditional marketing authorisation in March 2018. The drug was approved by the European Commission (EC) in May of the same year.
Clovis Oncology also submitted an application to the EMA seeking approval of Rubraca® as a maintenance therapy for patients with recurrent ovarian cancer in June 2018. Opinion from the CHMP on the application is expected by the end of 2018.
Clovis Oncology has sub-licensed the commercial development rights for Rubraca® outside the US and Japan to Servier.
Ovarian cancer and its symptoms
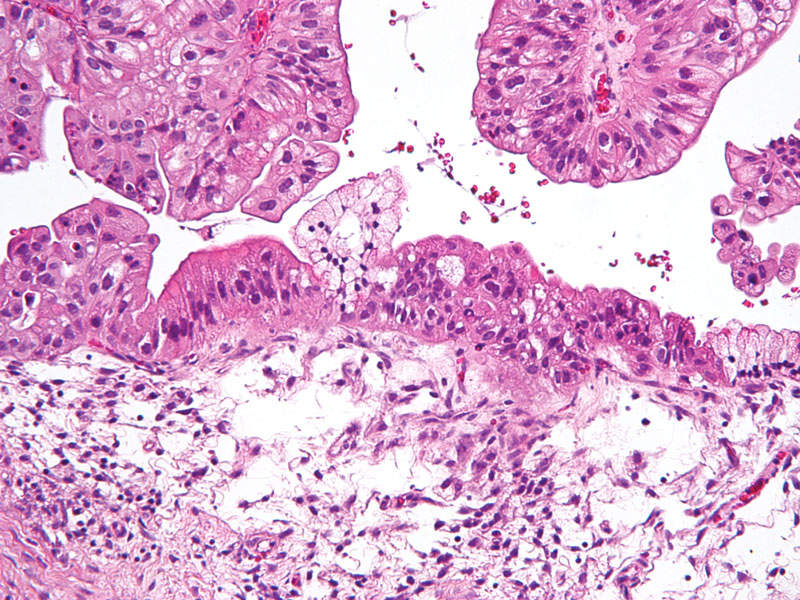
Ovarian cancer is one of the most difficult cancers to detect at an early stage, which is why most women who develop the disease are diagnosed only in the advanced stage. The disease accounts for approximately 3% of cancers among women, but has the highest fatality rate of any reproductive cancer.
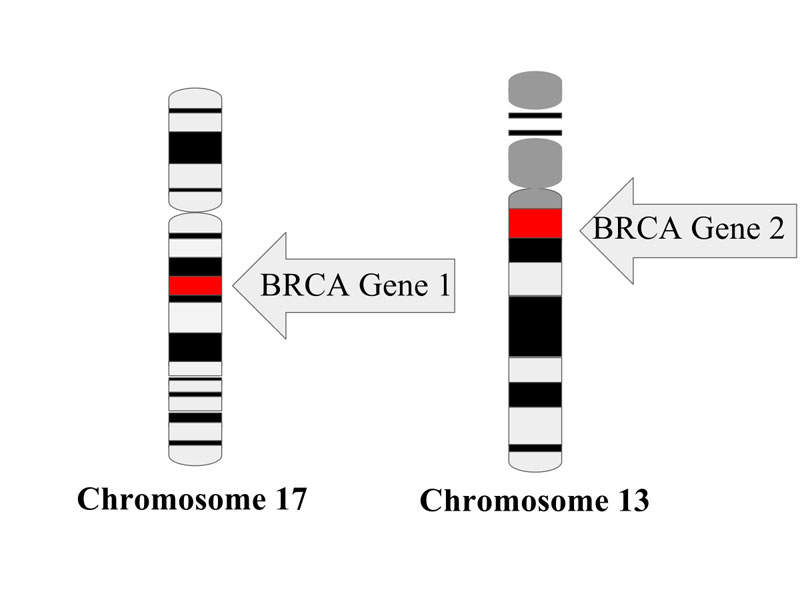
The inherited tumour suppressor genes BRCA1 and BRCA2 increase the risk of developing ovarian cancer. According to the American Cancer Society’s estimates, more than 22,000 women were diagnosed with the disease in the US in 2016.
It is estimated that roughly 39% of women who inherit BRCA1 mutations, and between 11% and 17% of women who inherit BRCA2 mutations, will develop ovarian cancer by the age of 70.
Common symptoms of the disease include continuous abdominal bloating, indigestion, nausea, loss of appetite, feelings of pressure in the pelvis, changes in bowel movements, increased abdominal girth and tiredness.
Rubraca’s mechanism of action
Rubraca® contains PARP-1, PARP-2, and PARP-3 inhibitor enzymes and can be used as a monotherapy for the treatment of patients with harmful BRCA mutations in advanced ovarian cancer patients who were earlier treated with two or more chemotherapies.
The drug restrains PARP enzymatic activity and increased formation of PARP-DNA complexes, which result in DNA damage, apoptosis, and cell death. It decreases tumour growth in cancer patients with or without deficiencies in BRCA.
Clinical trials on Rubraca
Clovis Oncology conducted clinical trials on Rubraca® in partnership with Foundation Medicine, which provided its FoundationFocus CDxBRCA test to select patients for Rubraca® treatment.
The FDA’s approval of Rubraca® and the FoundationFocus CDxBRCA test were based on two multi-centre, single-arm, open-label clinical trials, which evaluated the efficacy of Rubraca® in 106 patients with advanced ovarian cancer that had progressed after treatment with two or more prior chemotherapies.
Patients enrolled for the studies had BRCA1/2 status, which was determined through local germline BRCA test results or Foundation Medicine clinical trial assays.
All of the 106 patients were administered with Rubraca® 600mg twice a day. The objective response rate (ORR) and duration of response (DoR) were assessed by an independent radiology review (IRR) according to response evaluation criteria in solid tumours (RECIST) 1.1.
The results of the study showed that the response assessment by immune-related response (IRR) was 42%, with a median DoR of 6.7 months. The investigator-assessed ORR was 66% in platinum-sensitive patients, 25% in platinum-resistant patients, and 0% in platinum-refractory patients. The ORR was similar for patients with a BRCA1 or BRCA2 gene mutation.
In addition, the safety of Rubraca® was evaluated in 377 patients with advanced ovarian cancer. The most common adverse reactions included nausea, fatigue, vomiting, anaemia, abdominal pain, constipation, decreased appetite, diarrhoea, thrombocytopenia and dyspnoea.
Myelodysplastic syndrome (MDS) or acute myeloid leukaemia (AML) was reported in two of the 377 patients, while AML was reported in two patients with ovarian cancer enrolled in a blinded, randomised trial evaluating rucaparib versus placebo.