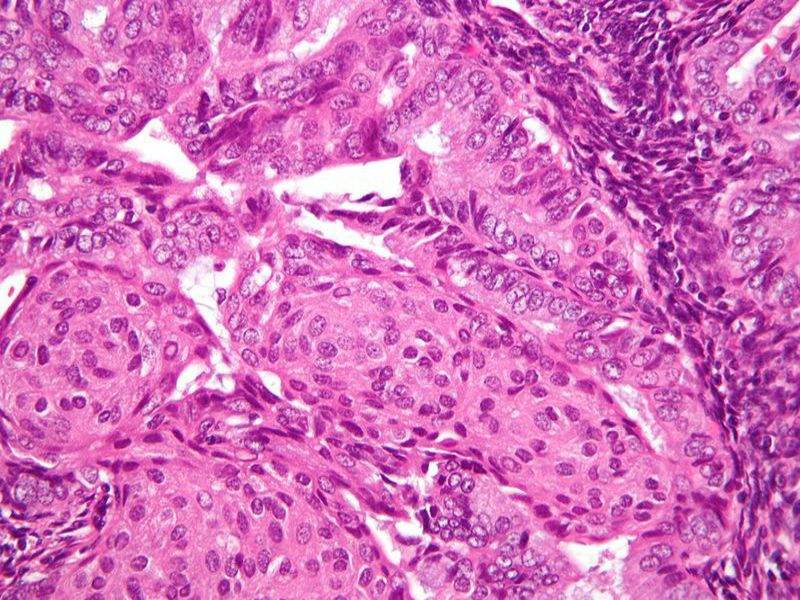

US-based biopharmaceutical firm Xenetic Biosciences has started patient enrolment in Phase II clinical trial of XBIO-101 (sodium cridanimod) and progestin therapy combination to treat patients with endometrial cancer.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
XBIO-101 is currently being developed as a small-molecule immunomodulator and interferon inducer.
The open-label, multi-centre, single-arm, two-period Phase II trial is designed to recruit a total of 72 recurrent or persistent endometrial cancer patients who have failed progestin monotherapy or are known to carry progesterone receptor negative (PrR) tumours.
Women established as PrR during the trial screening and those showing disease progression following progestin monotherapy for a minimum of four weeks will be administered with the investigational combination.
Xenetic Biosciences CEO Scott Maguire said: “We are very pleased to commence patient enrolment in this Phase II study with our flagship product candidate and believe this trial is designed to provide greater understanding of XBIO-101 for the treatment of progestin resistant endometrial cancer.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Based on these preliminary findings, we believe that XBIO-101 has the potential to increase the expression of PrR on endometrial tumours thereby making them more responsive to treatment with traditional progestin therapy, and ultimately provide a much-needed solution for late-stage endometrial cancer patients.”
The primary objective of the trial is to evaluate the anti-tumour activity of XBIO-101 in combination with progestin therapy measured by overall disease control rate (ODCR), while the secondary objectives are efficacy, safety and tolerability parameters.
The dosing of patients in the trial is expected to commence in the third quarter of this year.
Image: Micrograph of endometrioid endometrial adenocarcinoma. Photo: courtesy of Nephron.





