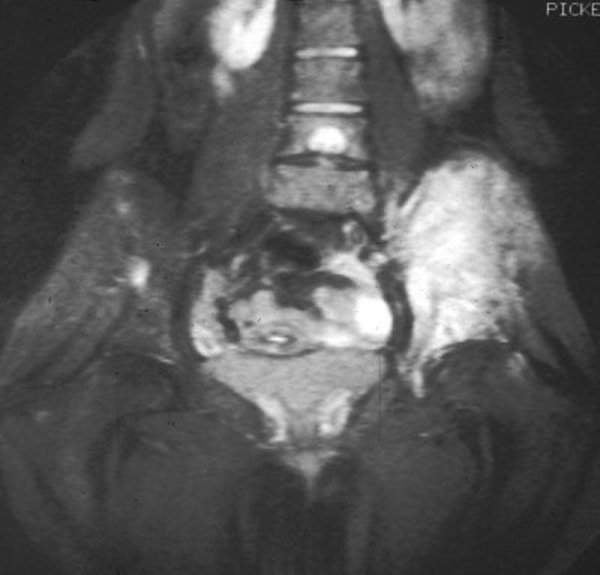

Oncternal Therapeutics begins dosing in Phase I trial of TK216 to treat Ewing sarcoma
US-based clinical-stage biotechnology company Oncternal Therapeutics began dosing patients in its Phase I clinical trial of TK216 to treat Ewing sarcoma.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Jointly developed by Oncternal and Georgetown University, TK216 is a small molecule that was designed to inhibit the biological activity of ets-family transcription factor oncoproteins in a variety of tumour types, resulting in the stoppage of cancer cell growth and tumour formation.
In Ewing sarcoma, TK216 targets a single and well-characterised genetic mutation responsible for the disease and inhibits the downstream effects of the chimeric protein EWS-FLI1 transcription factor.
Tilray and University of British Columbia begin Phase II trial of medical cannabis in Canada

Canadian-based medical cannabis producer Tilray and University of British Columbia began patient enrolment in Canada’s first Phase II clinical trial of medical cannabis to treat post-traumatic stress disorder (PTSD).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataCannabis plant belongs to the family of Cannabaceae of the nettle order, which is believed to be effective in soothing nausea, increase appetite, relieve pain and reduce epileptic seizures.
Claimed as one of the world’s first large-scale clinical trials, the Phase II, triple blind, placebo-controlled, randomised, crossover clinical trial is intended to determine the safety and efficacy of three potencies of medical cannabis to treat chronic, treatment-resistant PTSD symptoms resulting from a traumatic event.
AstraZeneca and Microsoft to develop new drug technology for cancer
AstraZeneca collaborated with Microsoft to develop new technology for drug intervention for a personalised treatment of cancer.
As part of the collaboration, the companies will design a new computer modelling system that will signal pathways in cancer cells to predict the best place for the application of new drugs.
AstraZeneca bioinformatics, oncology, innovative medicines and early development (IMED) principal scientist and global strategy lead Jonathan Dry believes that the new system will decrease the wet lab work and can pave the way for an accelerated drug development.
Novavax reports topline RSV F Vaccine data from two clinical trials in older adults
Novavax reported topline data from two clinical trials of its RSV F-protein recombinant nanoparticle vaccine candidate (RSV F Vaccine) in older adults.
The Resolve trial, which is a Phase 3 trial of the RSV F Vaccine in 11,856 adults who are 60 years of age and older, did not meet the pre-specified primary or the secondary efficacy objectives, and did not demonstrate vaccine efficacy.
The trial was a randomised, observer-blinded, placebo-controlled that was conducted at 60 sites in the US.
GSK reports positive results of phase three ZOE-70 trial of Shingrix to treat shingles

UK drug manufacturer GlaxoSmithKline (GSK) reported positive results from its phase three ZOE-70 study of Shingrix to treat shingles.
Shingrix has been developed as a non-live, adjuvanted, subunit (HZ/su) vaccine to prevent herpes zoster and its related complications.
Shingles is caused by the reactivation of latent chickenpox virus, triggering a painful, itchy rash on one side of the body.
GW Pharmaceuticals reports positive Phase III trial results of Epidiolex to treat Lennox-Gastaut syndrome
UK-based GW Pharmaceuticals reported positive data from its Phase III clinical trial of Epidiolex (cannabidiol or CBD) to treat seizures associated with Lennox-Gastaut syndrome (LGS).
Epidiolex was developed as a liquid formulation of pure plant-derived Cannabidiol (CBD) to treat various orphan pediatric epilepsy syndromes such as Dravet syndrome and Lennox-Gastaut syndrome.
The randomised, double-blind placebo-controlled Phase III trial enrolled 225 patients diagnosed with LGS aged between two and 55 years in its three study arms.
Destiny Pharma reports positive data from US clinical trial of exeporfinium chloride against SA bacteria
UK-based clinical stage biopharmaceutical company Destiny Pharma reported positive results from a US clinical trial of exeporfinium chloride to treat people with colonised nasal staphylococcus aureus (SA) bacteria.
Exeporfinium chloride (XF-73) is developed as a preventive anti-bacterial drug, which is a synthetic dicationic porphyrin derivative with antibacterial activity.
Sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the US National Institutes of Health, the two-stage clinical trial was conducted at the NIAID Clinical Trials Unit at Case Western Reserve University in Cleveland and at Anaheim Clinical Trials in California, US.
HSRx reports positive results from screening studies of HSRx 431 against Zika virus

US-based HSRx Biopharmaceutical reported positive results from studies testing the efficacy of HSRx 431 against Zika virus.
HSRx 431 was developed as an orally administered, broad-spectrum, antiviral drug candidate.
The screening studies conducted by SRI International in their Shenandoah Valley facilities, validated the efficacy of HSRx 431 in combating the Zika virus.
Orexo reports positive REZOLV study results of Zubsolv to treat opioid dependence
Swedish specialist pharmaceutical company Orexo reported positive results from its Retrospective Evaluation of Zubsolv Outcomes A Longitudinal View (REZOLV) retrospective study of Zubsolv to treat opioid dependence.
Zubsolv is composed of buprenorphine and naloxone and formed as a sublingual tablet (CIII) to treat opioid dependence.
With an enrolment of 1,080 patients, the REZOLV was the largest retrospective study, completed in the US to optimise treatment of opioid dependence.
The study intended to determine the efficacy of Zubsolv in treating opioid dependence.
Neurimmune reports positive Phase Ib PRIME results of aducanumab to treat Alzheimer's disease
Swiss-based biopharmaceutical company Neurimmune reported a positive outcome from its Phase Ib PRIME clinical trial of aducanumab to treat early stage Alzheimer's disease (AD).
Aducanumab has been developed in association with Biogen and is based on Neurimmune's patented Reverse Translational Medicine (RTM) technology platform.
It is developed as a human-recombinant monoclonal antibody derived from a de-identified library of B-cells and collected from healthy elderly subjects displaying no signs of cognitive impairment or cognitively impaired elderly people with unusually slow cognitive decline.





