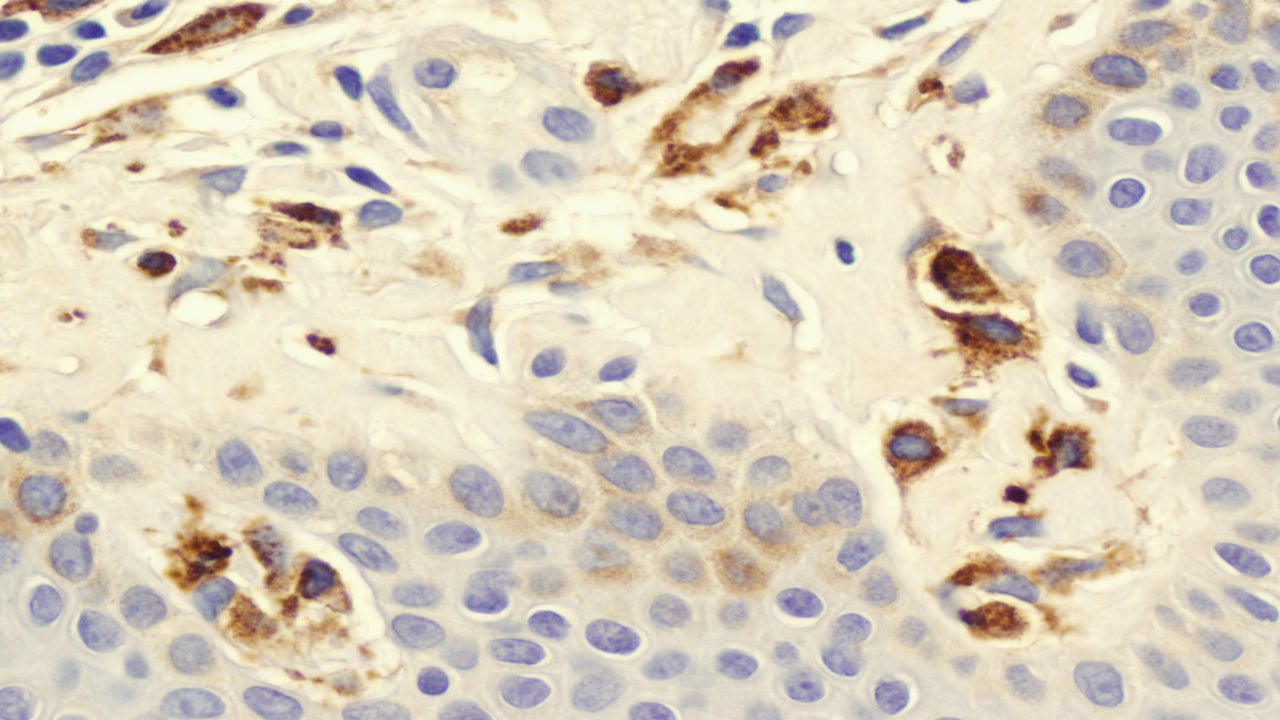
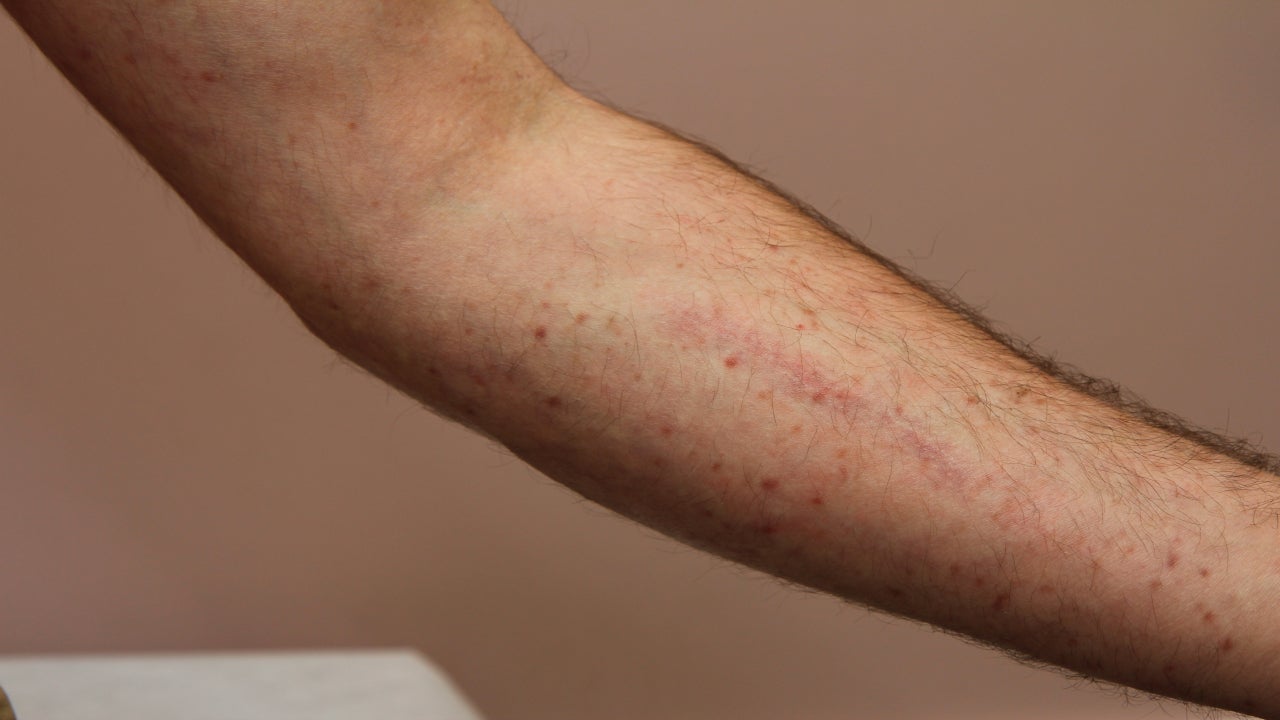
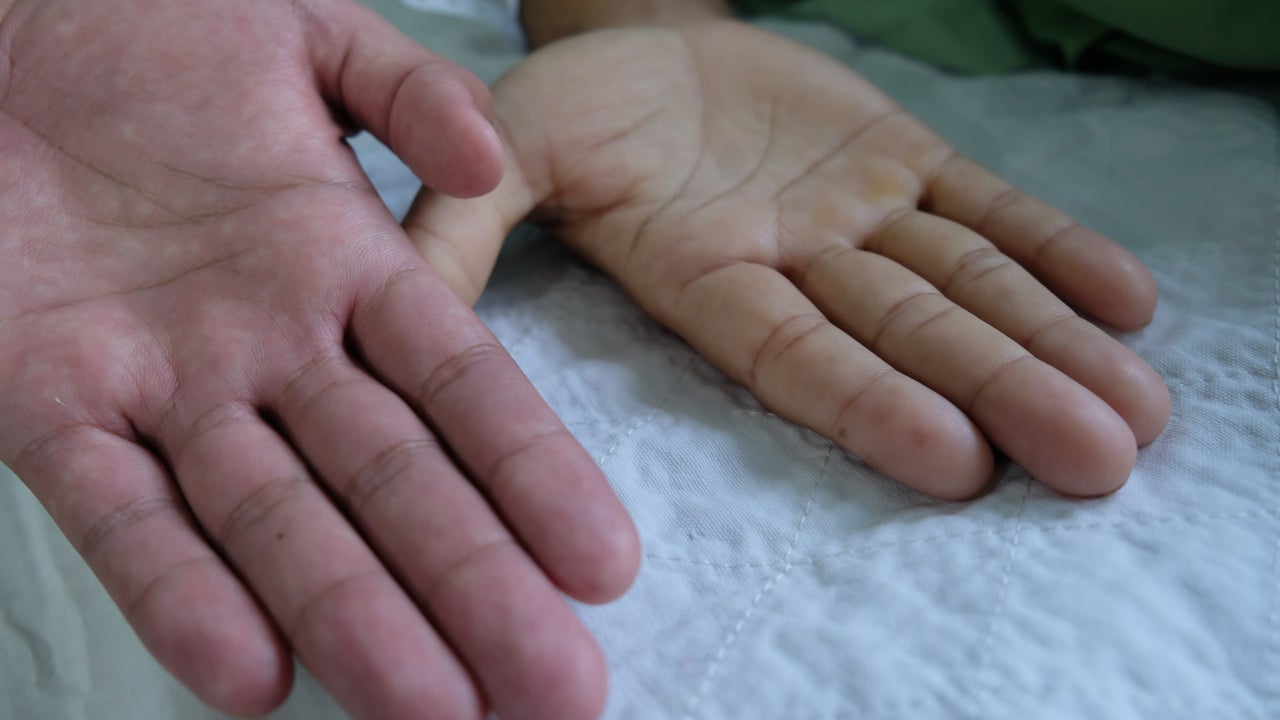
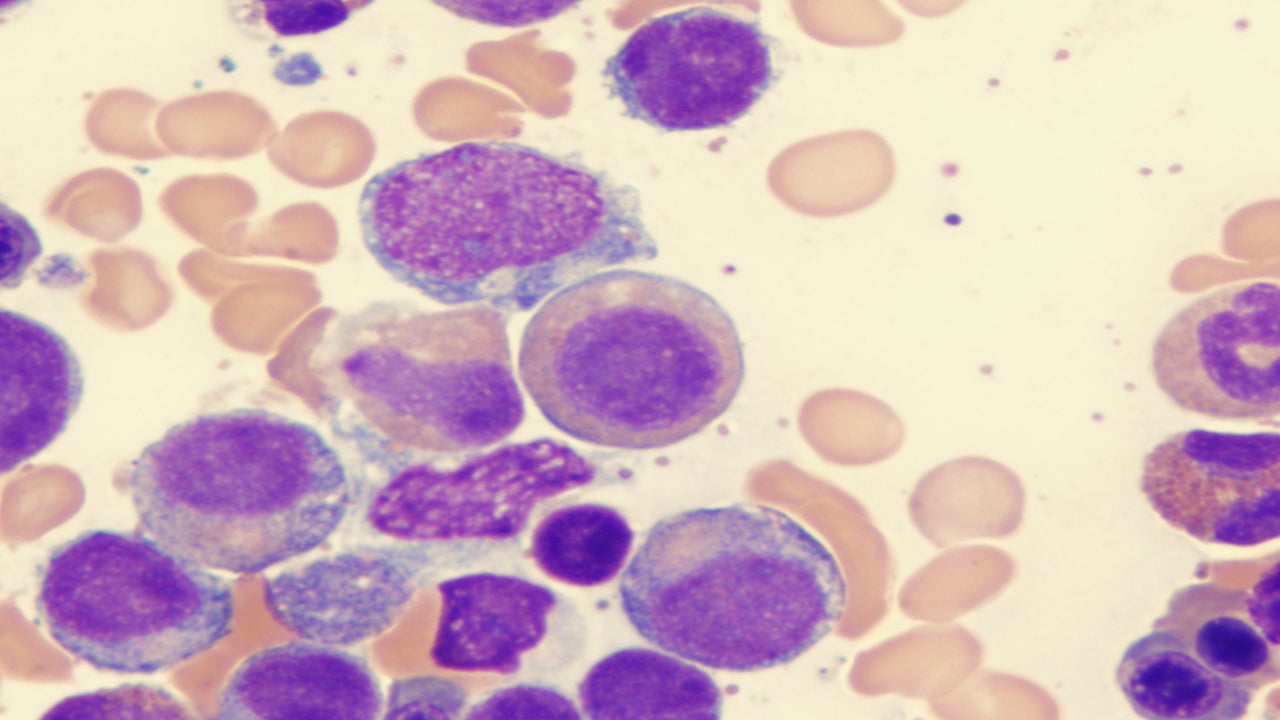
Blueprint’s Ayvakit expected to take Novartis’ Rydapt crown in advanced systemic mastocytosis but off-label use in indolent unlikely
Blueprint Medicines’ Ayvakit (avapritinib) is likely to displace its competitor, Novartis’ (SIX:NOVN) Rydapt (midostaurin), in advanced systemic mastocytosis (AdSM), experts…