With pressure mounting to shorten timelines and tighten oversight, Flex Databases is betting on a single, configurable eClinical platform to help sponsors and contract research organizations (CROs) tame fragmented data, de‑risk the trial master file (eTMF), and industrialize risk‑based monitoring. Global Director of Business Development Marietta Sarkisian explains why a human‑in‑the‑loop AI “co‑pilot,” modular entry points for smaller organizations, and deep localization will be critical to keeping trials compliant, affordable, and globally connected over the next three to five years.
Flex Databases is an all-in-one eClinical platform that won in three categories in the 2025 Clinical Trials Arena Excellence Awards.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Clinical Trials Arena (CTA): Flex Databases has just won awards for Innovation, Product Launches, and Business Expansion. How do you interpret this recognition for the company’s current stage and direction?

Marietta Sarkisian: First of all, thank you for the acknowledgement and the awards. We are truly flattered. Second, I suppose these awards are a real reflection of the achievements we have made in recent years. We had a huge leap in our product development, and we surely keep moving forward with ambitious goals on our minds.
CTA: Sponsors and CROs still struggle with fragmented systems, slow study start-up, and eTMF inconsistencies. Which of these issues do you see as most damaging to timelines today, and why?
Marietta Sarkisian: All of the issues mentioned are actually part of a bigger puzzle, which is the absence of a robust and unified eClinical system in place. Fragmented data always appears when there is a disruption in data entry – be it due to the lack of a system or the use of multiple different systems to cover a single process. The speed of study start-up is usually one of the most common reasons of significant budget increases, so for me, investing in a robust system at the very beginning helps keep budgets as planned and prevents unexpected increases. Yet it is still challenging to convince some companies of the need for digitalization even today. The good thing is – the majority has already acknowledged the need of having CTMS and eTMF at least to manage the study start-up efficiently. The last part – eTMF inconsistency – is the most critical, I would say. At the end of the day – your eTMF is the most important outcome of your study, having any inconsistency in it may actually lead to the failure of the whole study. So I would say the last is the most damaging though the first two do not make lives of clinical trial experts easier either.
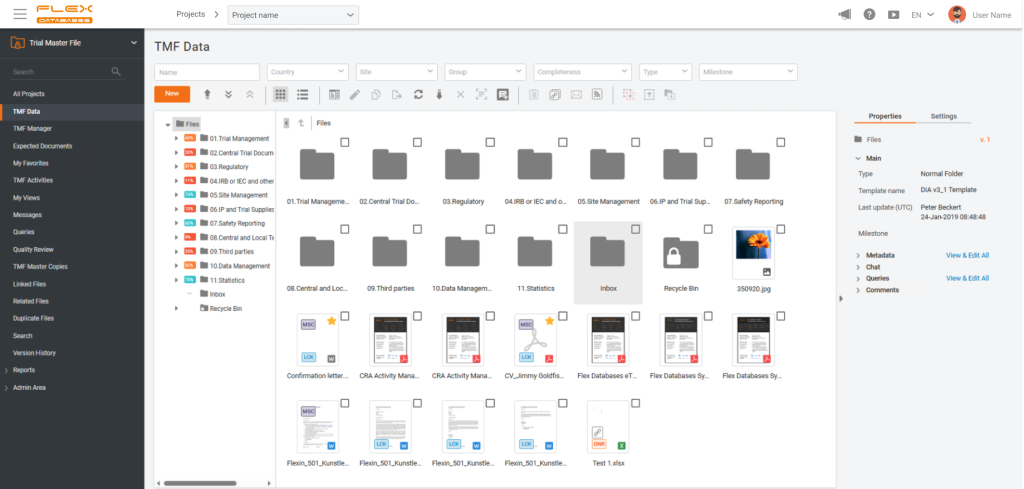
CTA: You invested early in confidence-scored AI and human-in-the-loop eTMF automation. What drove that decision, and how has real-world use confirmed or reshaped your thinking?
Marietta Sarkisian: This is a great question to be honest. First of all, implementing AI into our eTMF was already on the surface since there’s been a huge buzz of using AI in the industry for quite a while. So the most time-consuming and, let’s call it, boring work is filing the documents. Yet at the same time, this is a very important activity from a compliance perspective activity within a clinical study. So the decision to make it more automated, though still human-controlled, was brought by our product team and quickly supported by the company’s leadership.
As for the industry adoption of this feature, surely it is in the early days but we already have positive feedback from the users and I am sure in 5-7 years this functionality will become a core feature of any advanced system in the industry.
CTA: AI in GxP environments raises understandable concerns. What were the hardest questions you had to address from regulators, auditors, or client QA/IT teams before they were comfortable?
Marietta Sarkisian: The most challenging part was definitely AI validation. When we started dealing with it, there were no detailed guidelines available. We leveraged our extensive GxP experience and in‑depth knowledge of regulations to ensure that AI‑powered systems were thoroughly validated.
CTA: With the co‑pilot model, automation does the heavy lifting while humans stay in control. How are eTMF team roles changing in organizations that adopt this approach?
Marietta Sarkisian: I cannot judge in depth from the client’s perspective, but I’d say it does not change roles as much as it changes the burden for eTMF managers. They now spend less time on low‑value tasks such as filing artifacts correctly in the eTMF structure and assigning metadata that is already contained in the documents. So far AI does not influence the workflow or the roles, but it definitely speeds up the initial step of document upload and distribution.
CTA: You stress region-hosted AI, private processing, and full GxP/21 CFR Part 11 validation. How do you personally balance speed of innovation with the need for validation and data sovereignty?
Marietta Sarkisian: It’s all about professional teamwork. While some of us are in constant creative search, others provide support and ensure full compliance.
CTA: Flex Databases is a single native platform rather than a stack of acquired tools. What were the key architectural or strategic choices that made that possible?
Marietta Sarkisian: Keeping our core product values in mind. We are a flexible, unified, and hassle-free solution meaning the whole logic of our system is based on the fact that the system should easily adapt to clients’ processes and not vice versa. So you will not find two identical twins of Flex Databases’ system because each client’s environment is set to be the way they need – their own workflows, templates, access rights. Everything is fully adjustable. At the same time these adjustments do not require any dramatic efforts. Any system owner from the client’s end can build the workflow or assign a new access level without having any technical background. It gives us this hassle-free approach covered. And finally – the system is scalable though the more the merrier. The modules are interconnected. Seamless data flow from one part of the system to another not just saves time but minimizes the chances of human errors too and simply takes pressure of manual tasks from the clinical teams, allowing them to focus on what really matters.
CTA: Many organizations are tired of juggling multiple platforms and vendors. When someone is considering consolidating onto Flex Databases, what main hesitations do you hear, and how do you address them?
Marietta Sarkisian: To be completely honest, the only hesitation we hear is related to the initial investment required. Because whoever has enough investments will almost always prefer all-in-one system rather than handling a bunch of vendors and making their teams suffer from/struggle with conquering a bunch of systems instead of one. But for small biotech companies, CROs, and under-funded academic institutions, the thing I constantly hear is – we want it all but we cannot afford it. This is the only possible disadvantage. But since Flex Databases has a scalable/modular approach, it is still not a problem. Depending on the clients needs I can always recommend what module to start with. For example, if this is a small biotech, I would definitely advise going for our eTMF since it can cover the majority of their needs, and for a CRO depending on whether they are using sponsors’ eTMF or they need to include theirs into the bidding, I would sometimes recommend adoption of CTMS first to have their most important part of services covered and compliant. So there is always a way to start with something smaller and grow it into a vast system covering the whole study life cycle.
CTA: Your suite covers CTMS, eTMF, EDC, PV, QMS, LMS, and finance. How do you decide what to build natively versus integrate, and how do you avoid spreading your focus too thin?
Marietta Sarkisian: This is a very tricky question, and I would say as always, the demand is dictating the supply. Since the majority of our clients are using our eTMF, I would say this is a heavily invested and feature-rich module in Flex Databases, followed by our CTMS. Yet we have a lot of clients who are more than happy of using our PV system along with QMS and LMS. So it always varies from the needs of our clients, and the market demand.
And here is a good example of our EDC revival – we have been keeping it in a sleeping mode for some time and went on with a lot of integrations of our CTMS, PV, eTMF and the EDC systems used by our clients. But lately more and more clients started asking us about bringing EDC back to light since again – everybody wants to have an all-in-one system. So we brought our EDC back from its sleeping mode and even strengthened it with a Risk-Based Monitoring module.
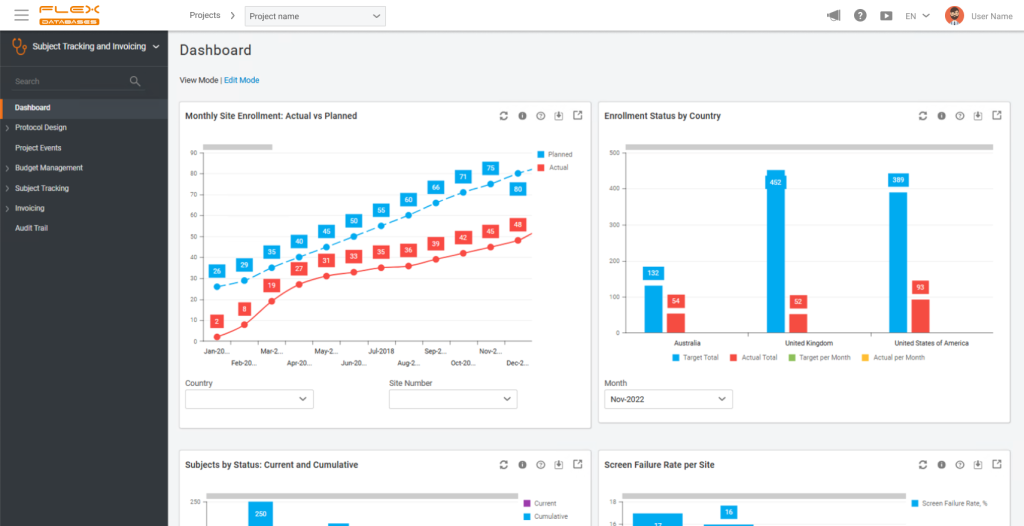
CTA: Risk-Based Monitoring is now embedded into your platform. How do you expect RBM to change monitoring strategies and site relationships in the near term?
Marietta Sarkisian: I hope to see a change in the approach to monitoring planning with the tool in place. On one hand, everybody is mostly thinking about increasing the number of visits to sites with unsatisfying results – such as a higher number of deviations or screen failures, etc. On the other hand, I would also focus on reducing the number of visits to sites that perform well. This approach not only makes the budget more efficient and reduces the burden of unnecessary monitoring visits from project teams, but also lead to more trusting relationships between the sponsors/CROs and sites.
CTA: Looking at your expansion into Saudi Arabia, what did you learn about winning trust and adoption in a fast-evolving but still emerging research market?
Marietta Sarkisian: This year we had a significant interest in our system in this region and gained clients in Saudi Arabia who are now actively using our all-in-one system. But winning trust in this region is a challenging task indeed. It is a very closed area where reputation means everything. So winning the first contract there opened the doors to new opportunities and led to another contract in just a couple of months in between.
Apart from clinical trial regulations business practices in Saudi Arabia differ from those in Europe and the US. So we adapted quickly to establish the contracts in alignment with all the requirements.
CTA: Arabic localization and SFDA alignment go beyond just language. How do you approach regulatory and cultural localization so that it becomes a real advantage for clients?
Marietta Sarkisian: This is the advantage of having a truly flexible solution. If the system is flexible enough to adjust, then you do not have to worry about building it from the scratch for each country’s requirements. So this is actually the key. On top of that we are proud to be one of the few eClinical systems that can be installed on the client’s premises. Meaning, when we were approached by a Saudi state-governed institute we were able to meet the requirement of capturing the data on the institute’s servers. It is a unique service that leads to a more complicated implementation but gives the client the opportunity to use an advanced and highly developed system of the industry rather than to be suffering from building an in-house solution with zero experience and stretched timelines.
CTA: Emerging research hubs want to be fully integrated into global trial portfolios. What role do you see Flex Databases playing in making that integration a reality?
Marietta Sarkisian: I would like to think of our system as a bridge for the region to stay connected to the most developed clinical trial hubs in the world. At the end of the day, we all work in strict accordance with ICH GCP despite the location, and this is exactly what makes it feasible using the same system in any part of the world. Especially in case of our system being flexible enough to be adapted to any local requirements.
CTA: Over the next three to five years, where do you expect the next major operational bottlenecks in clinical trials, and how are you positioning the platform to stay ahead of them?
Marietta Sarkisian: Over the next 3-5 years, the biggest bottlenecks will be site capacity/start-up delays, recruitment and retention for narrower cohorts, and fragmented data with higher audit/traceability demands, all worsened by protocol complexity. We’re positioning the platform to connect and automate core workflows end-to-end – speeding start-up, making feasibility more realistic, unifying data with lineage and reconciliation, and using human-in-the-loop automation to cut cycle times without sacrificing quality.

CTA: Thank you, Marietta, for sharing such a grounded view of how Flex Databases is approaching AI, platform unification, and regional growth. Your perspective on balancing flexibility, compliance, and affordability will resonate with many of our readers navigating similar pressures in today’s clinical trials landscape.
About Marietta Sarkisian
Marietta Sarkisian has over 13 years of experience in the clinical trials industry. Marietta has held roles in both operations and business development across global markets. She brings a strong understanding of clinical research and a strategic approach to delivering tech-driven solutions.





