Flex Databases won the Innovation, Product Launches, and Business Expansion awards in the 2025 Clinical Trials Arena Excellence Awards for advances that address persistent bottlenecks in clinical trial operations.
Many sponsors and CROs continue to struggle with challenges such as fragmented systems, inconsistent trial master file (TMF) metadata, slow study start-up, and cross-functional misalignment – issues that directly delay trial timelines and increase operational risk.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Flex Databases won the Innovation award for advancing TMF automation with AI-driven efficiency and controls suited to regulated environments. The company won the Product Launches award for introducing a new module in their unified eClinical platform that reduces silos and improves end-to-end study orchestration. It won the Business Expansion award for executing a regulation-aligned extension into Saudi Arabia that enables cross-border trial management across the Gulf.
TMF Automation: Confidence-Scored AI, Human-in-the-Loop Controls, and Validated Compliance
Flex Databases’ eTMF automation addresses the everyday realities of document overload by taking on the tasks that typically slow teams down—classification, filing, and metadata capture. When documents arrive via upload, email, or integration, the system identifies the document type, places it in the appropriate eTMF location, and extracts metadata such as dates, sites, and countries directly from content. This reduces manual input and produces metadata that is consistent and useful for search and oversight.
Winning across innovation, product launches, and regional expansion highlights Flex Databases’ maturity as both a technology provider and a global operational partner. It demonstrates the company’s ability not only to build advanced tools but also to deploy them at scale across complex regulatory environments.
A key differentiator is the confidence-scoring approach. Each automated action is scored; high-confidence classifications and metadata assignments proceed automatically, while lower-confidence cases are flagged for review according to client-defined thresholds. This human-in-the-loop “co-pilot” model embeds risk management into daily operations, ensuring users retain control where it matters. It supports accuracy without returning teams to fully manual routine, and it provides a clear audit trail of what AI did and what a human verified.
Compliance and security are built into the deployment. The AI runs on client-dedicated, private infrastructure hosted in the client’s chosen region, and data is not shared externally. The feature set is delivered with GxP and 21 CFR Part 11 validation documentation, including requirements traceability, IQ/OQ and UAT materials, and vendor qualification artifacts. This makes it feasible for quality and IT teams to assess and accept the controls without bespoke, ad hoc validation work. Automated consistency checks help keep the TMF inspection-ready by catching metadata deviations early rather than relying on late-cycle cleanups. Beyond efficiency, the enriched, context-aware metadata increases the value of TMF analytics, enabling more reliable completeness checks, status tracking, and trend identification across sites or study phases.
Unified eClinical platform: CTMS, eTMF, EDC, PV, and site tools now strengthened by RBM

“I have been with Agenus for over 26 years. Over those 26 years, our Flex CTMS and TMF implementation is the smoothest and fastest implementation of a validated IT system we have done.”
– Craig Winter, CIO, Agenus Inc.
Flex Databases’ platform brings multiple core functions – clinical trial management system (CTMS), eTMF, electronic data capture (EDC), pharmacovigilance (PV), learning and quality management system (QMS) – onto a shared data model. The shared backbone reduces duplication and reconciliations that often occur when separate systems exchange partial or delayed data. In practice, study start-up can move faster when site, document, training, and activation tasks are orchestrated in one environment, and close-out is more predictable when data and documentation align without manual stitching across tools.
Unlike multi-system ecosystems assembled through acquisitions, Flex Databases’ platform is built on a single native architecture. This removes data sync delays, integration uncertainties, and version drift that frequently occur across stitched-together solutions.
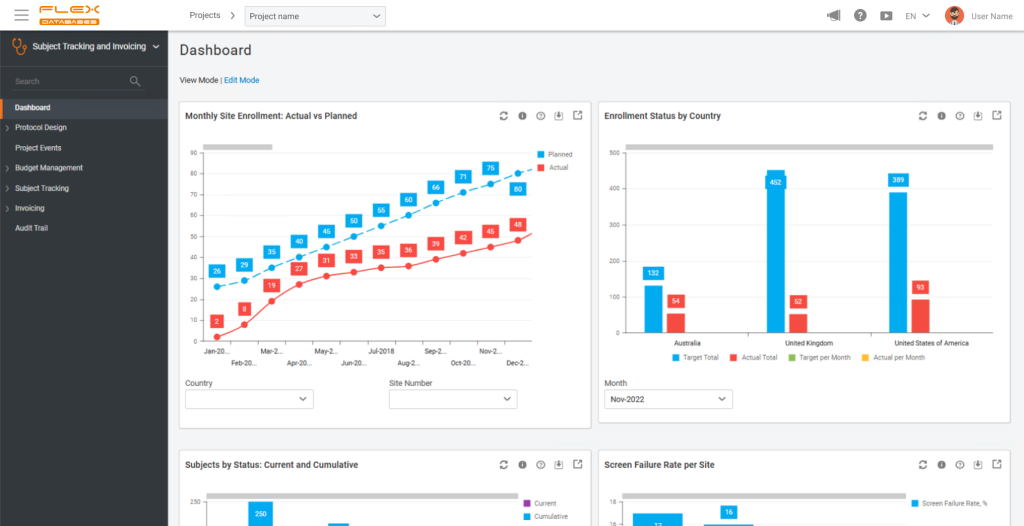
Decision-making improves when operational and clinical data is visible across modules in real time. For example, monitoring visit report preparation compliance can be viewed alongside site performance, or oversight of adverse reactions can be managed via the same system in both Pharmacovigilance and EDC. This cross-functional view shortens feedback loops and helps teams address issues before they accumulate. From a governance perspective, working with a single vendor and validating an integrated platform can reduce total cost of ownership compared with maintaining and validating multiple point solutions. The platform’s configurable localization and role-based controls support different organizational structures and geographies without re-architecting the system, making it adaptable for sponsors, CROs, and sites operating at varying scales.
Saudi Arabia entry
Flex Databases’ entry into Saudi Arabia reflects the growing demand for modern, compliant clinical operations technology in the region. The company’s traction with multiple clients shows that its platform addresses the needs of organizations seeking to improve oversight, data quality, and operational efficiency in an increasingly competitive research environment. Alignment with international standards such as ICH GCP and 21 CFR Part 11 supports audit readiness and helps local teams meet the expectations of global sponsors and CROs.
Establishing a regional presence also creates broader operational advantages. Cross-border study management across Gulf countries becomes simpler when processes and data models are unified on a single platform. This matters as markets such as the UAE, Qatar, and Bahrain continue strengthening their clinical research capabilities. A consistent system reduces the complexity of multinational trial execution, improves interoperability between sites, and supports centralized oversight. It also enables faster knowledge transfer, helping teams in neighboring markets adopt validated workflows without rebuilding processes.
Crucially, regional standardization enhances collaboration with international partners. When local sites operate on modern, compliant systems, study startup and execution require fewer adjustments and can progress more quickly. By supporting the Gulf region with a platform built for scalability, data integrity, and global regulatory expectations, Flex Databases contributes to elevating the region’s readiness for complex, data-driven clinical research.
Together, these capabilities position Flex Databases as one of the most comprehensive and operationally mature eClinical ecosystems in the industry – built for organizations that prioritize compliance, automation, and real-time visibility across global studies.

“Clinical research teams are under constant pressure to deliver quality results with limited time and resources. Technology only matters when it removes real barriers for clinical teams. Our goal is to give sponsors, CROs, academic institutions – all our partners the tools that simplify work, strengthen compliance, and move studies forward faster. These awards reflect our commitment to building practical, intelligent solutions that make clinical trials more efficient.”
– Marietta Sarkisyan, Global Director of Business Development
Company Profile
Flex Databases is an all-in-one eClinical platform that centralizes every major process required to plan, conduct, and manage clinical trials. Designed for sponsors, CROs, and research sites of all sizes, its unified system replaces fragmented tools with a single secure workspace that improves visibility, compliance, and operational efficiency across global studies.
The company’s platform includes a comprehensive suite of modules:
CTMS (Clinical Trial Management System)
- Site & study management: study setup, site activation, site initiation, monitoring visit schedules.
- CRA activity management: visit trackers, confirmations/follow-ups, visit reports.
- Offline site-visit reporting (CRA app/offline mode).
- Subject tracking: enrollment, inclusion/exclusion, visit windows, screen failures.
- Investigators & sites database: site profiles, feasibility data, contacts, regulatory docs.
- Custom fields / configurable trackers / roles & permissions.
- Cross-project reporting, BI dashboards, KPI tracking.
- Built-in invoicing/site-payments linked to visits and milestones.
- Integration links to eTMF for one-click filing of visit documents.
- Advanced feasibility scoring, site performance scoring, study-level templates, capacity planning.
eTMF (electronic Trial Master File)
- DIA-aligned TMF structure, versioning, audit trail, e-signatures, GxP / Part 11 readiness.
- Flexible folder/templates, metadata configuration, QC workflows.
- Upload methods: drag-and-drop, e-mail upload, batch imports, WebDAV / external drive options.
- Smart search, completeness trackers, expiry/renewal alerts.
- One-click filing from CTMS (visit reports, letters).
- Real-time completeness dashboards for inspection readiness.
- Document OCR / auto-indexing, AI-assisted classification
AI in eTMF
- AI document classification: the system automatically detects the correct TMF zone and classification for uploaded documents.
- AI metadata assignment: auto-populates fields such as document title, type, date, site, country, and study-level metadata.
- AI-assisted quality review: helps detect incomplete fields, missing metadata, and potential filing inconsistencies.
- AI-accelerated bulk filing: dramatically reduces manual document sorting and speeds up TMF clean-up.
EDC (Electronic Data Capture)
- Rapid, low/no-code eCRF builder (drag-and-drop).
- Real-time edit checks/validation, ePRO and eConsent support.
- RTSM / randomization and drug supply features.
- Export & data standards: CDISC-ready exports and audit-ready data.
- Compliance: GCP, 21 CFR Part 11, HIPAA where applicable.
- Fast study build timelines
- RBM dashboards and central monitoring support integrated with CTMS/eTMF.
- Advanced data transformations, API pipelines to external stats teams, extended ePRO device integrations.
Pharmacovigilance (PV / Safety)
- ICSR case intake and full case lifecycle management.
- MedDRA coding support, duplicate detection, audit trail.
- E2B compatible XML reporting and regulatory submission support.
- Signal detection, line listings, case follow-up workflow
Quality Management System (QMS)
- SOP and controlled document management, version control, review/approval workflows with e-signatures.
- CAPA, deviations, incident logging, audit scheduling and tracking.
- Risk assessments and compliance dashboards.
- Role-based QMS workflows tied to studies and organizational structure.
- Integrated audit trails that cross-reference TMF / EDC entries for root cause investigations.
Learning Management System (LMS)
- Course creation (self-study and trainer led), video/docs attachment, quizzes.
- Training matrices per role/project, certificate generation, overdue training alerts.
- Training status dashboards and exports for audit.
- Integration with CTMS for study-specific training assignments.
- SCORM import/export and third-party content connectors.
Project Budgeting, Finance & Invoicing
- Budget setup, cost tracking, forecasting, and multi-project financial reporting.
- Site payment rules, visit/milestone triggers for invoicing, and exportable finance reports.
- Automated subject visit payments and reconciliation workflows.
- Direct integrations to accounting systems (SAP/QuickBooks) or bank payment gateways.
With interconnection across all modules, Flex Databases eliminates data silos and accelerates data-driven decision-making. Compliance with ICH E6(R3) / GCP, 21 CFR Part 11, EU Annex 11, GDPR, HIPAA-ready deployments, ISO/IEC 27001 (certified), ISO 9001 references, GAMP5 best-practice alignment, ALCOA++ data-integrity adherence, CDISC-compatible exports, E2B-R3 (PV reporting) support, and standard validation artefacts (URS, IQ/OQ, UAT, traceability matrices) – designed to keep systems secure, validated and inspection-ready.
With Flex Databases, clinical research teams gain control, automation, and complete operational visibility – all within one powerful eClinical ecosystem.
Contact Details
Links
Website: https://flexdatabases.com/





