WCG, a tech-enabled partner with over 55 years’ experience accelerating clinical research while safeguarding participant safety, won the Innovation Award in the Ethics Review category in the 2025 Clinical Trials Arena Excellence Awards.
It won the award for WCG ClinSphere® eReview Manager, their solution which modernizes Institutional Review Board (IRB) review through collaborative, quality-focused workflows, delivering measurable gains in speed, quality, and operational visibility for sponsors, contract research organization (CROs), sites, and institutions.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.

Reengineering IRB review for faster decisions without sacrificing rigor
eReview Manager replaces email handoffs with a shared, real‑time collaborative workspace that keeps reviewers and study teams aligned. Instead of generic intake, its smart, protocol‑aware questionnaires surface only what is relevant to the submission type and study design, reducing duplicate data entry and limiting opportunities for error.
Completeness indicators and guided navigation help teams finish submissions in fewer steps, translating into shorter prep time without weakening documentation standards. A defined pre‑review stage within eReview Manager acts as a quality gate: WCG staff and clients can clarify inconsistencies, confirm required attachments, and correct missing details before materials reach the IRB. This step increases first‑pass completeness and reduces rework, which, in turn, accelerates the path to determination. The practical effect is fewer cycles between submitters and the board and a more predictable timeline from intake to decision.
Modern document handling and operational visibility
Document logistics are a known bottleneck in IRB operations. eReview Manager addresses this with structured tagging, batching, and bulk actions that minimize manual downloading and filing. Standardized outcome‑document names make final materials easier to recognize and route for internal records, audits, and site communications. These mechanics matter at scale, where even small reductions in handling time compound across multi‑site portfolios.
Equally important is the live view of progress. eReview Manager’s real‑time status tracking, filterable reports, and exportable data give sponsors, CROs, and sites the ability to see where each submission stands, which tasks are outstanding, and where delays are emerging. With this visibility, study leaders can intervene earlier—whether that means supplying a missing document, reallocating internal resources, or sequencing site activations more effectively. The result is a shift from reactive follow‑ups to proactive portfolio management, supporting more reliable startup plans.
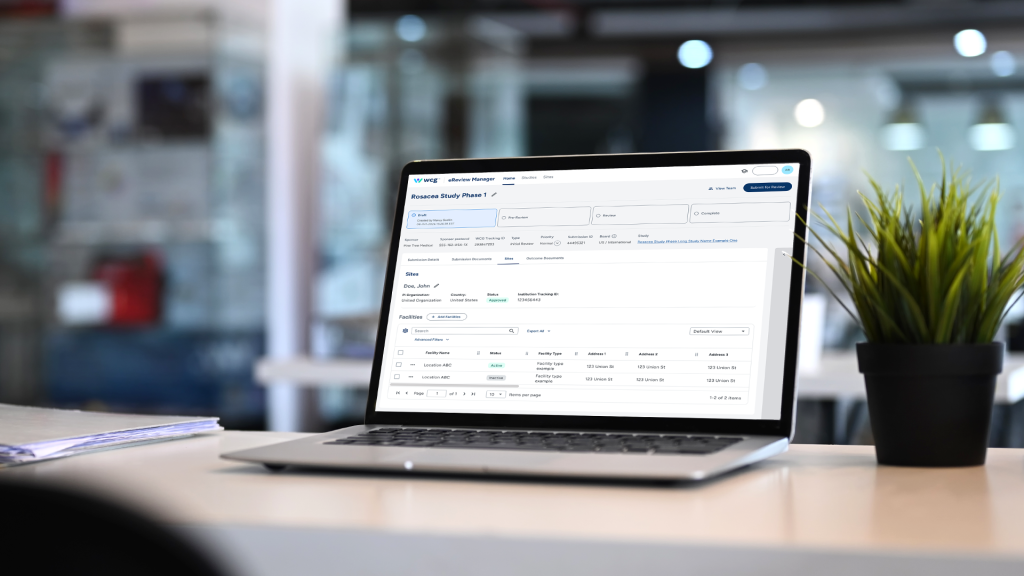
Collaboration anchored in expert governance
eReview Manager’s collaboration hub centralizes dialogue with WCG’s IRB experts inside the platform, replacing scattered email chains with a persistent record of questions, clarifications, and resolutions. This reduces the back‑and‑forth that often elongates timelines and helps teams maintain context when staff change or multiple stakeholders contribute. The chat history and role‑based submission management features also support intra‑team coordination, so sponsors, CROs, and sites can align on who provides which inputs and when.
Crucially, these efficiencies are paired with established governance. Submissions within eReview Manager proceed to WCG’s AAHRPP‑accredited, ISO 9001‑certified board, bringing independence and quality controls to the process. By uniting a modern collaboration hub with an accredited review function, WCG delivers faster movement through the operational steps while preserving the rigor and traceability expected in ethics review. This balance of speed and oversight supports WCG’s selection for the Innovation in Ethics Review award.

“We are truly honored to receive this Innovation award for ClinSphere eReview Manager. This recognition underscores WCG’s role as a trusted partner in empowering the clinical research community with next-generation, technology-enabled solutions. By consolidating IRB submission and expert review into a seamless, collaborative platform, we are setting a new benchmark for efficiency and safety. Our vision is to enable sponsors, CROs, and sites to advance innovative therapies more swiftly and ultimately improve patient outcomes.”
– Sam Srivastava, CEO, WCG
Company Profile
WCG is at the forefront of accelerating clinical research worldwide, serving as the trusted and preferred partner to biopharmaceutical and medical device companies, contract research organizations (CROs), research institutions, and site partners. Offering a unique combination of expertise, next-generation data and insights, and tech-enabled solutions, WCG reduces complexity and optimizes study operations and outcomes while maintaining the highest standards of human participant protection. For more than 55 years, WCG has maintained a relentless commitment to efficiency, safety, and impact, empowering clinical trials to deliver life-improving therapies swiftly.
Contact Details
Carmin Gade, PhD, Chief Marketing Officer, WCG
Links
Website: https://www.wcgclinical.com/
ClinSphere eReview Manager: https://www.wcgclinical.com/technologies/ereview-manager/



